Indigo System CAT RX
Coronary Mechanical Thrombectomy
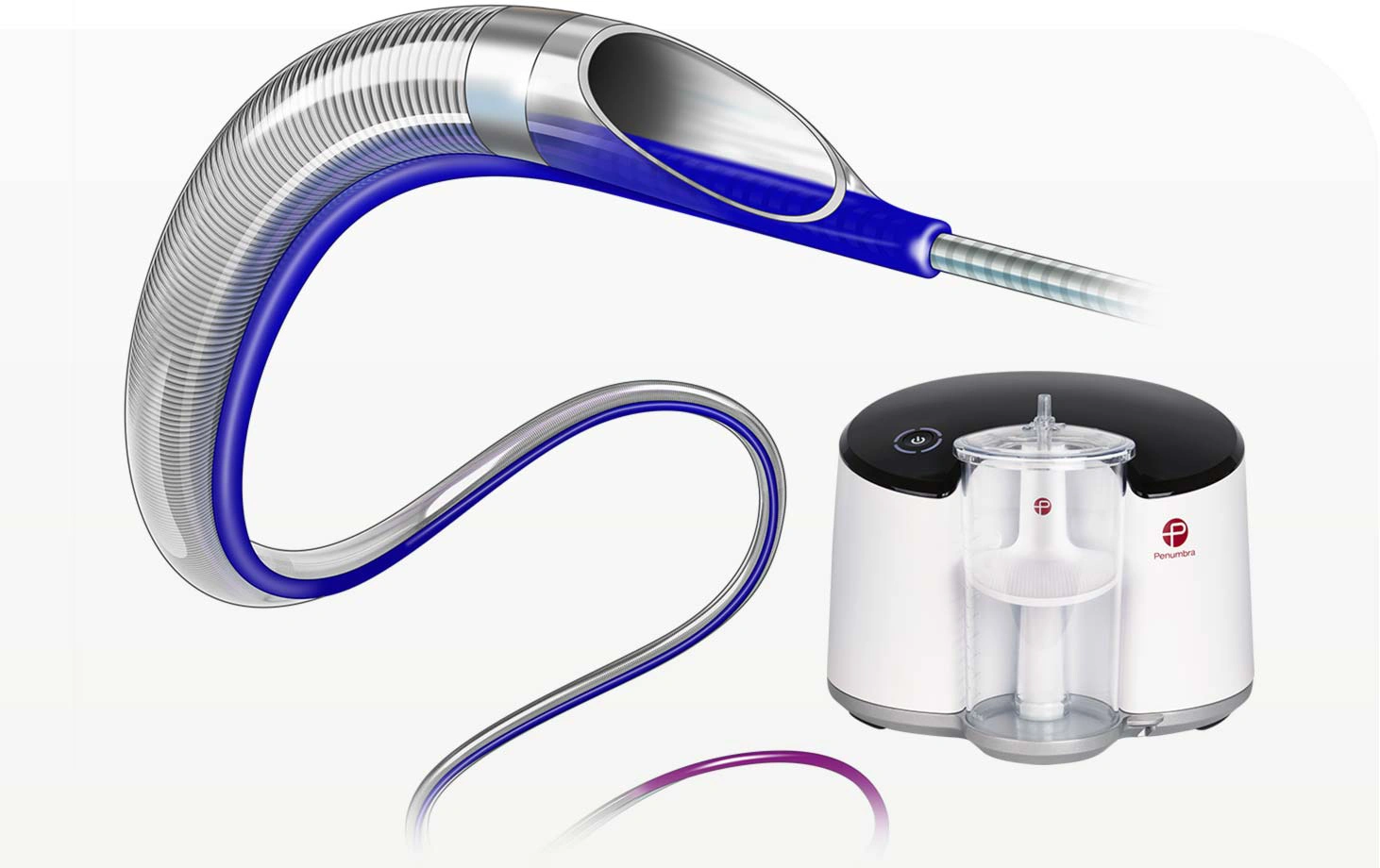
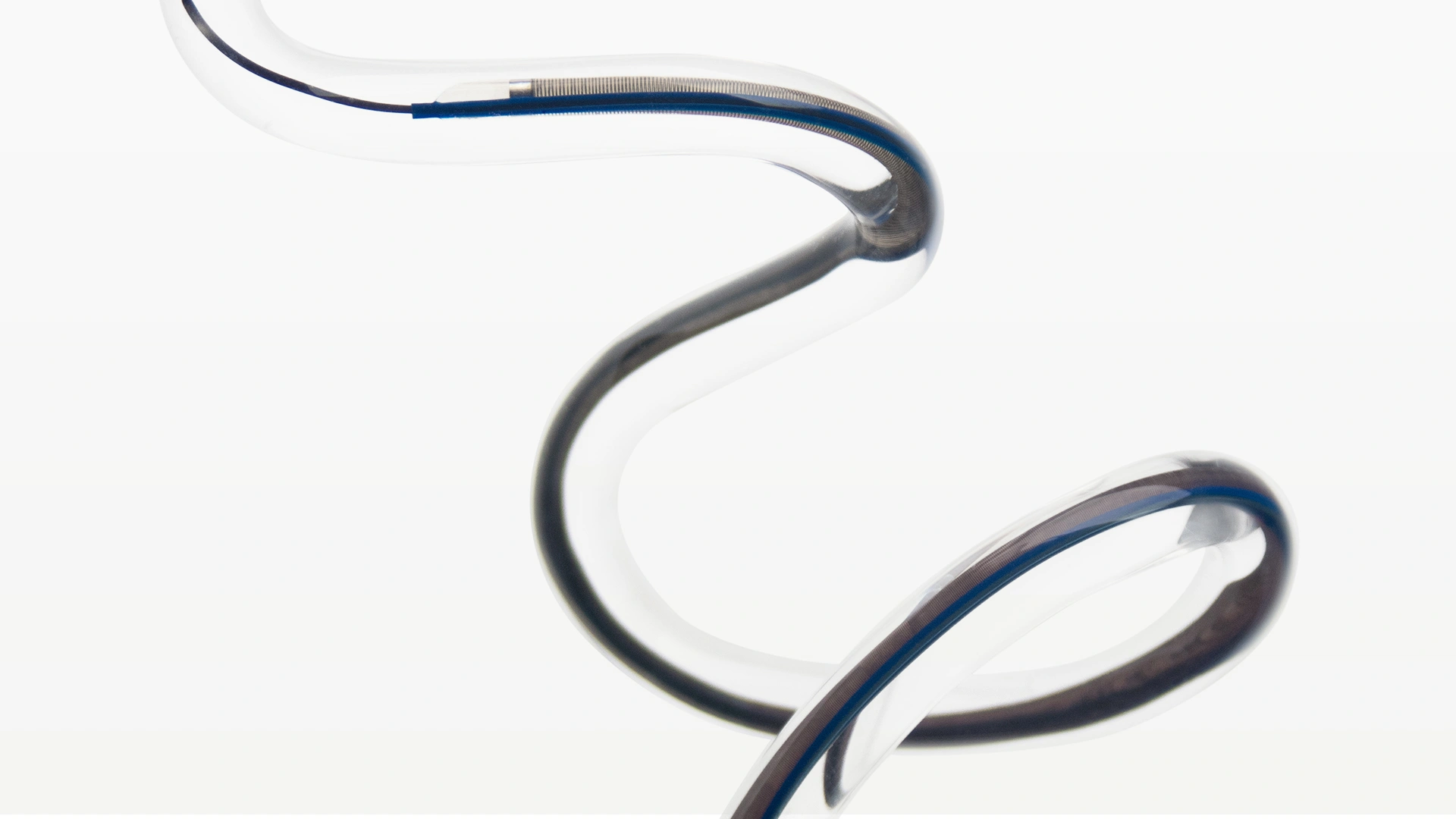
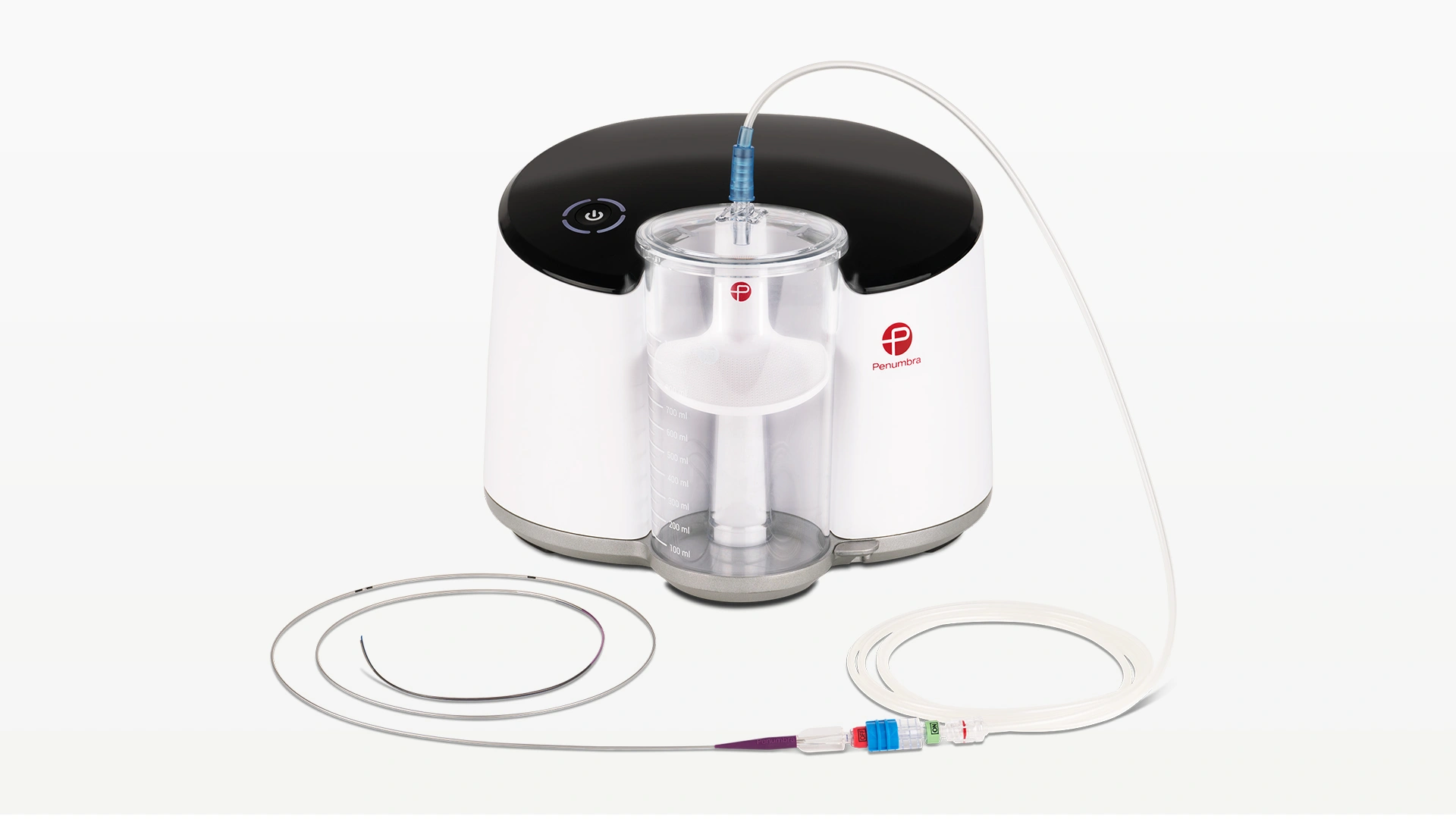
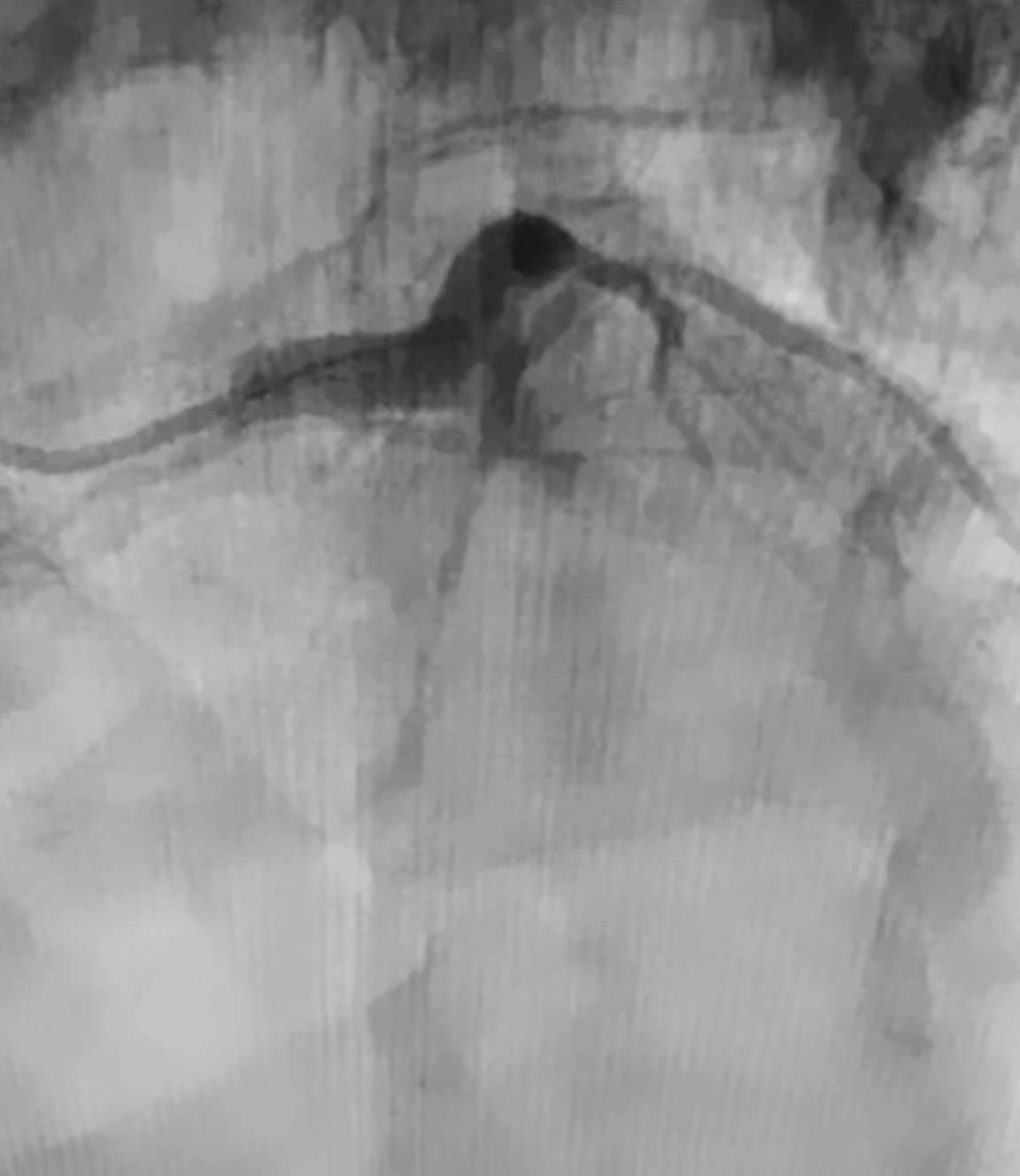
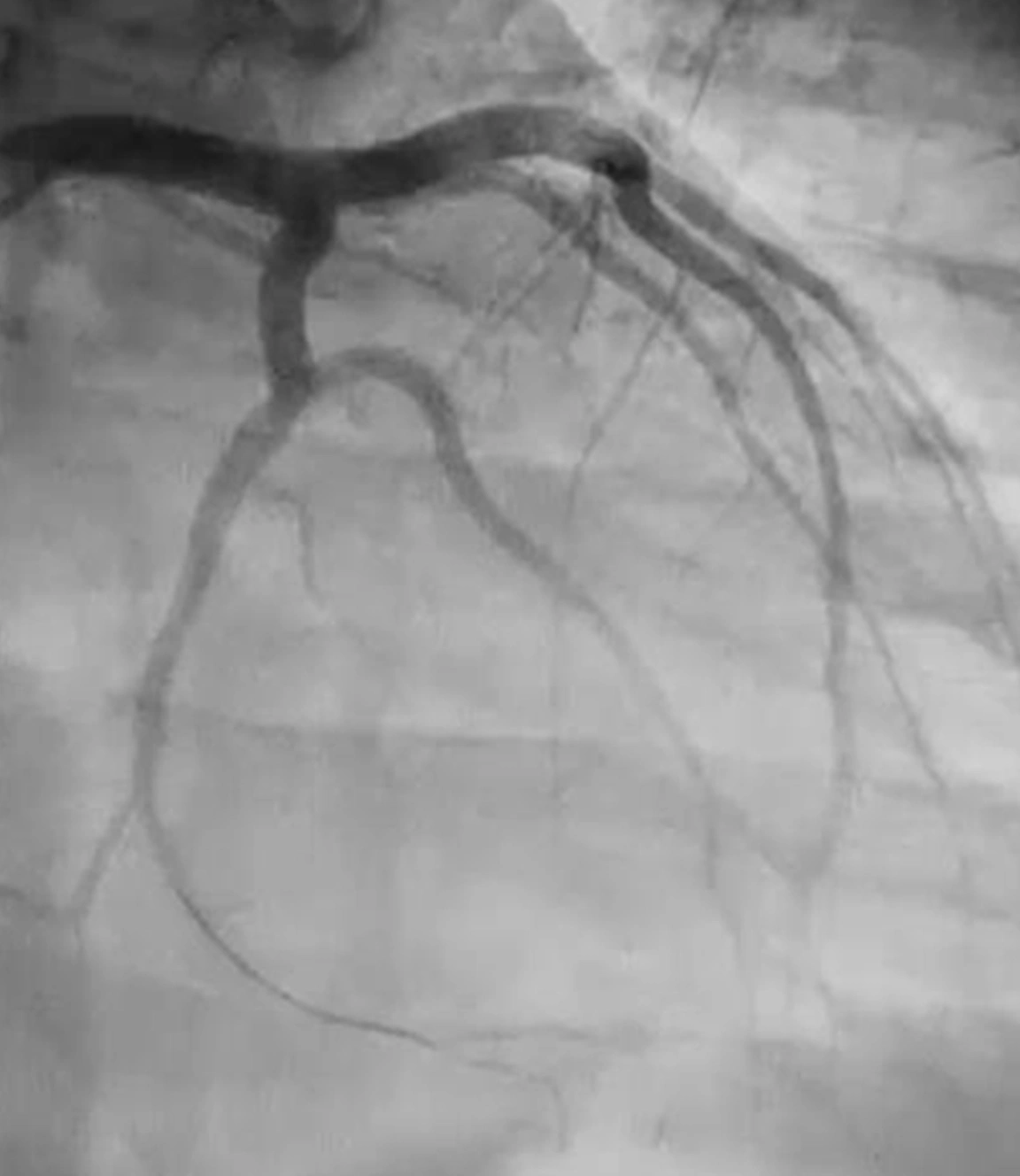
Penumbra introduced the Indigo™ System CAT™ RX for the removal of fresh, soft emboli and thrombi from the coronary and peripheral vasculature. Engineered with neuro-tracking technology, CAT RX is designed to navigate tortuous anatomy and track to distal coronary vasculature while maintaining sustained mechanical aspiration with the Penumbra ENGINE™.
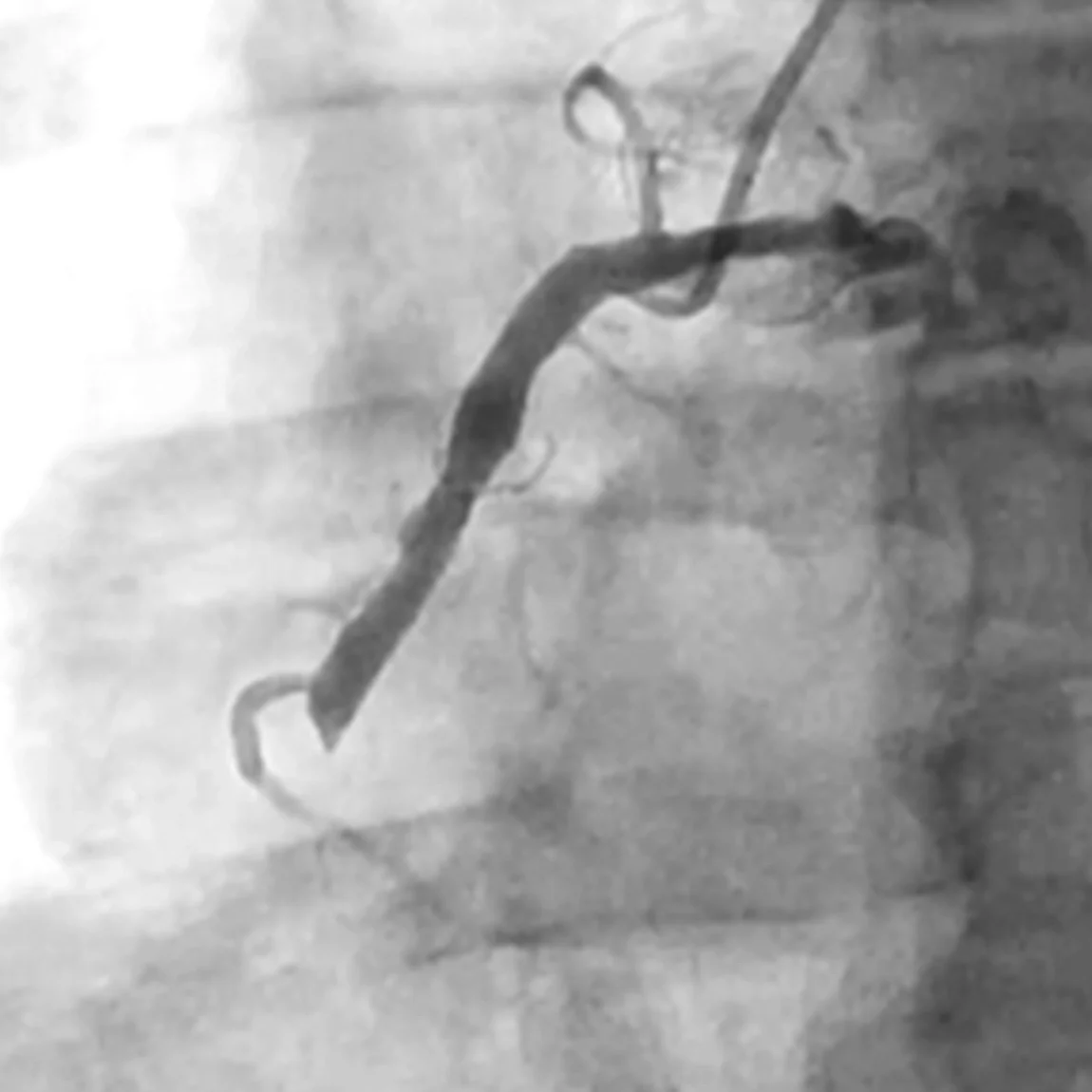
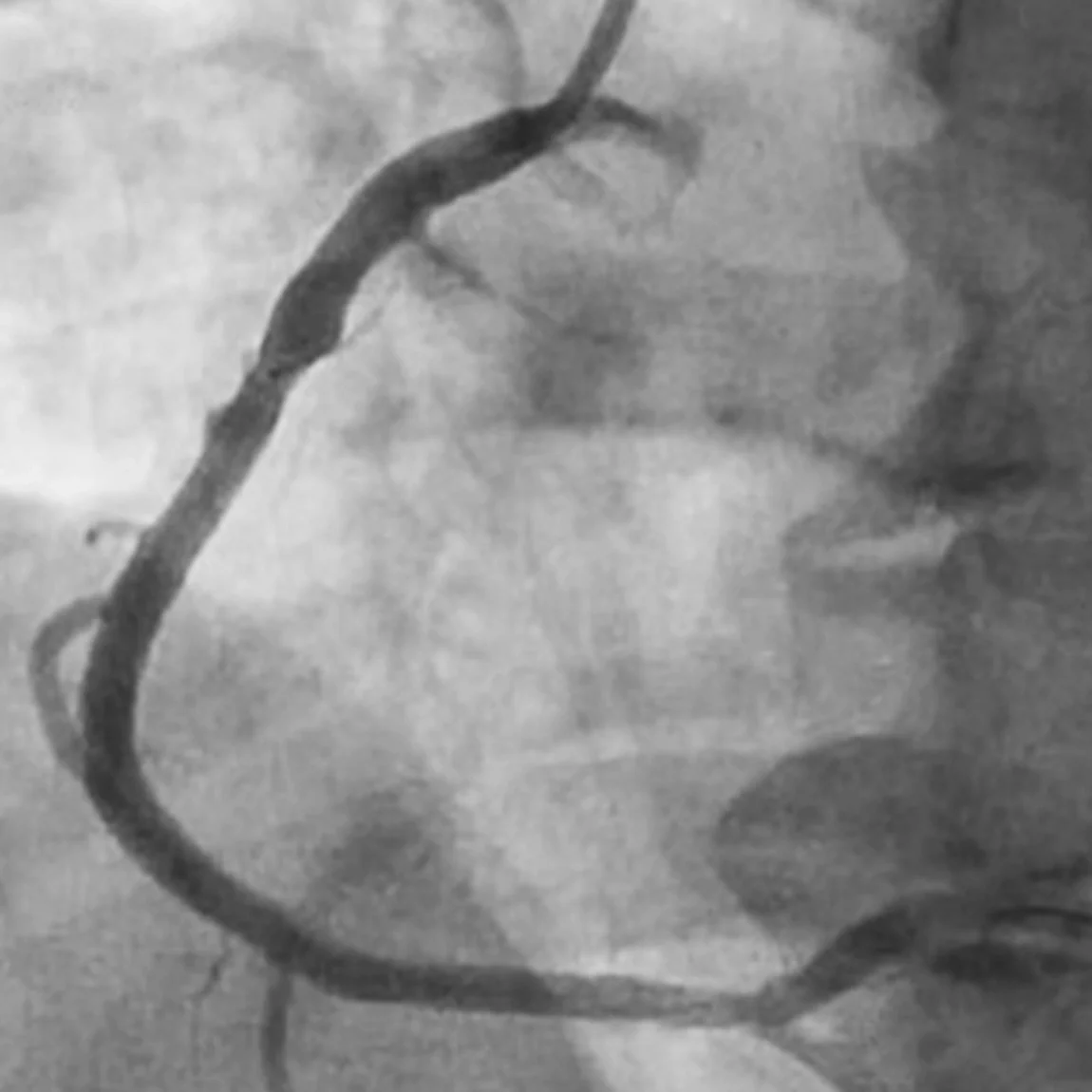
The latest CHEETAH Study was a prospective, multicenter study evaluating the safety and performance of CAT RX1 in patients with a high thrombus burden acute coronary vessel occlusion prior to PCI. CHEETAH met its primary endpoint while demonstrating high technical success and safety with improved TIMI flow, myocardial perfusion and no device-related SAEs (including stroke). Learn more about the CHEETAH study results here: CHEETAH Study Results
1. Precaution: The safety and effectiveness of this device for use in the treatment of ST-Elevation Myocardial Infarction (STEMI) has not been established. Complications from the use of this device in this manner could lead to death, permanent impairment, and/or the need for emergency medical intervention.
-
- Advanced trackability with neuro-tracking technology
- Enhanced deliverability with proximal laser-cut hypotube
- Large aspiration lumen for maximised clot engagement
- Powered by Penumbra ENGINE, designed to maximise aspiration for powerful, deep vacuum
-
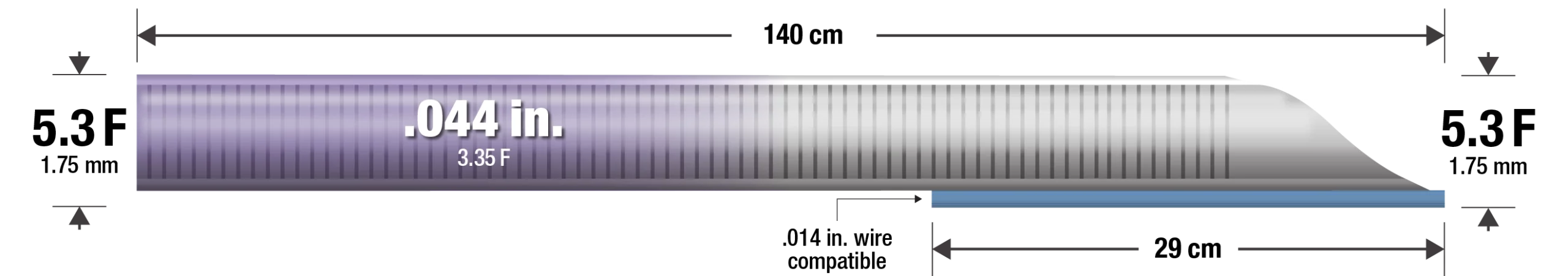
- 5.3 F (1.75 mm), 140 cm length
- 29 cm rapid exchange segment
- .014″ wire compatible
- 6 F Guide Compatible (.070″ ID or larger)
- Compatible with Penumbra ENGINE Aspiration Source and Penumbra ENGINE Canister
- 5.3 F (1.75 mm), 140 cm length
CHEETAH Study Results
Indigo System CAT RX Features & Data
New Evidence Supports Continuous Power Aspiration with Indigo System CAT RX
CIT, May 2022
Product Gallery
Please refer to Penumbra's image license for use of materials
Related Cases
Images used with permission and provided for illustrative purposes only. Procedural techniques and decisions based on physician’s medical judgment. Individual results may vary. Consents on file at Penumbra, Inc.
Related Products
Resources
-

-

-

Therapies & Conditions
Learn more about how our products are used for a broad spectrum of conditions.
-

Request a Demo
Want to see Penumbra products in action? Request a demonstration for your facility.