The S-BAT Info Center
Science-Based Aspiration Thrombectomy
by Penumbra, Inc.
What is Science-Based Aspiration Thrombectomy
(S-BAT)?
S-BAT encapsulates the fundamental scientific principles utilized in the development of safe and effective aspiration thrombectomy technology and procedural techniques.
Since Penumbra’s inception in 2004, these principles have existed at the core of the company’s rapid technology advancement and continue to inform future developments. In establishing S-BAT, Penumbra seeks to build a community of thought leaders dedicated to protecting the integrity of safe and meaningful innovation in stroke care through scientific objectivity.
WATCH: Science-Based Aspiration Thrombectomy with Dr. Ian Kaminsky and Dave Barry for SLICE Symposium
Principles of S-BAT
-
-
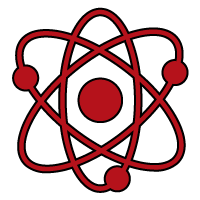
2. Vessel Sizing
Understanding average vessel sizing and the optimal catheter-to-vessel ratio for effective aspiration
-

3. Flow Control
Evaluating the consequences of excessively restricting blood flow during a thrombectomy procedure
-
4. Time
Analyzing procedure time in relation to patient outcomes, and key opportunities to reduce time
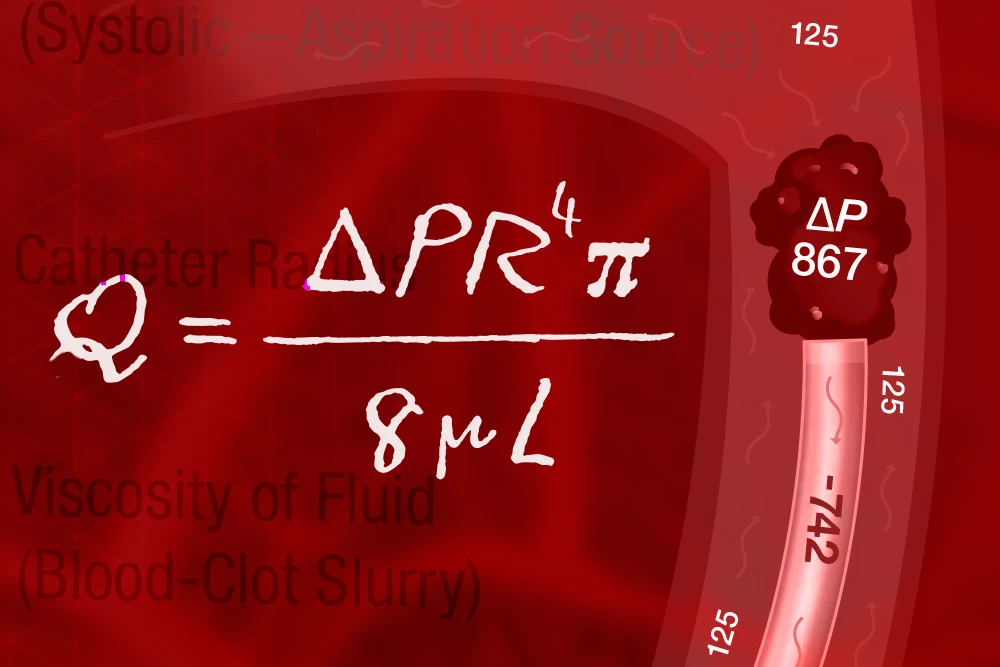
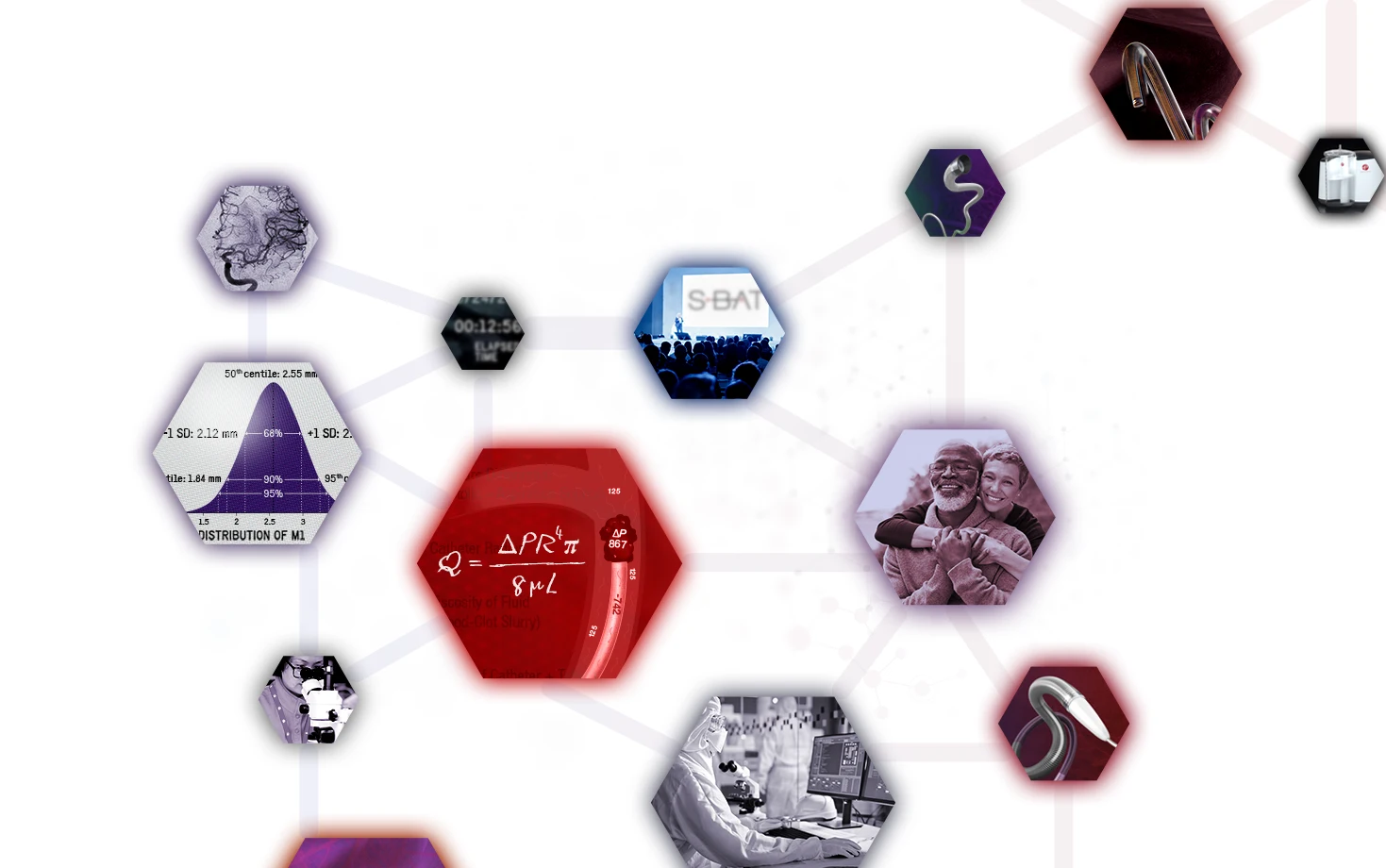
1. Physics:
Recognizing energy flows and what actually occurs when catheter meets clot
Understanding and Applying Pressure Differentials in Aspiration Thrombectomy
The science of aspiration explores how the fundamental physical laws that govern the universe apply in the setting of aspiration thrombectomy. It characterizes the interaction between the catheter size and the absolute difference in pressure between the device and blood vessel at the site of the occlusion, making clear how both under-sizing and over-sizing a catheter can lead to sub-optimal results.
The reason for this can be distilled down to a simple concept: High pressure always flows to low pressure. While this may seem straightforward, it is often overlooked during device selection.
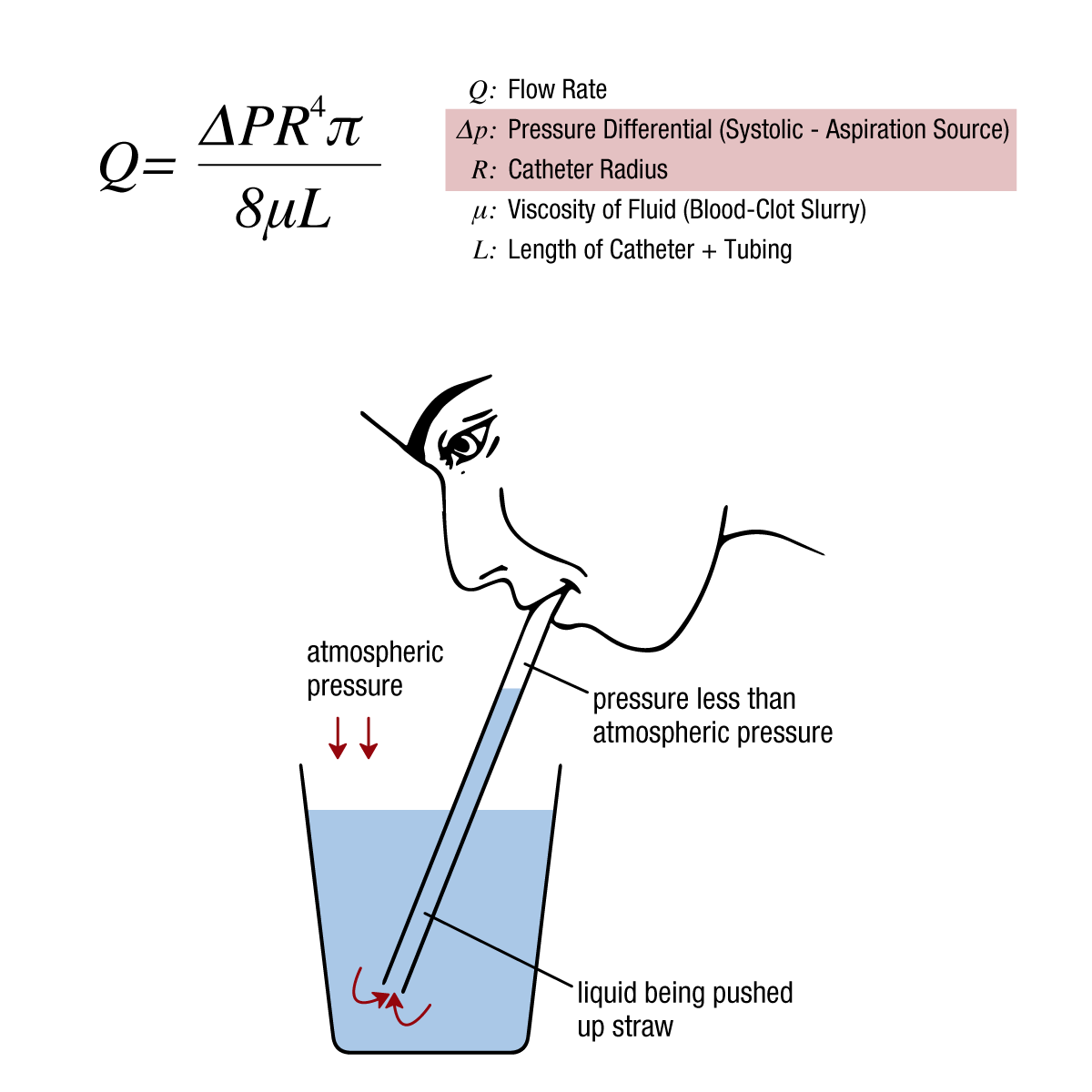

Figure 1: The Hagen-Poiseuille equation for fluid dynamics can be applied to a variety of everyday scenarios. In aspiration thrombectomy, it allows us to control 2 key variables: the catheter radius/diameter and the pressure differential, in order to maximize ingestion rates.
Myth: Fluid is pulled when a low pressure environment is introduced (i.e., a straw sucks liquid). Truth: Fluid is pushed by high atmospheric pressure to the low pressure environment.
How does this apply to aspiration thrombectomy?
In aspiration thrombectomy, many are conditioned to believe that the suction from a vacuum is the catalyst for effective aspiration, leading to the assumption that a larger catheter lumen and a reduction in the opposing systolic pressure may aid in clot removal.
However, the opposite is true. Systolic pressure is critical for clot engagement and removal. Without exposure to adequate systolic pressure, clot is unable to benefit from a pressure differential, handicapping the aspiration catheter’s ability to “push” the clot to the lower pressure environment (the canister).
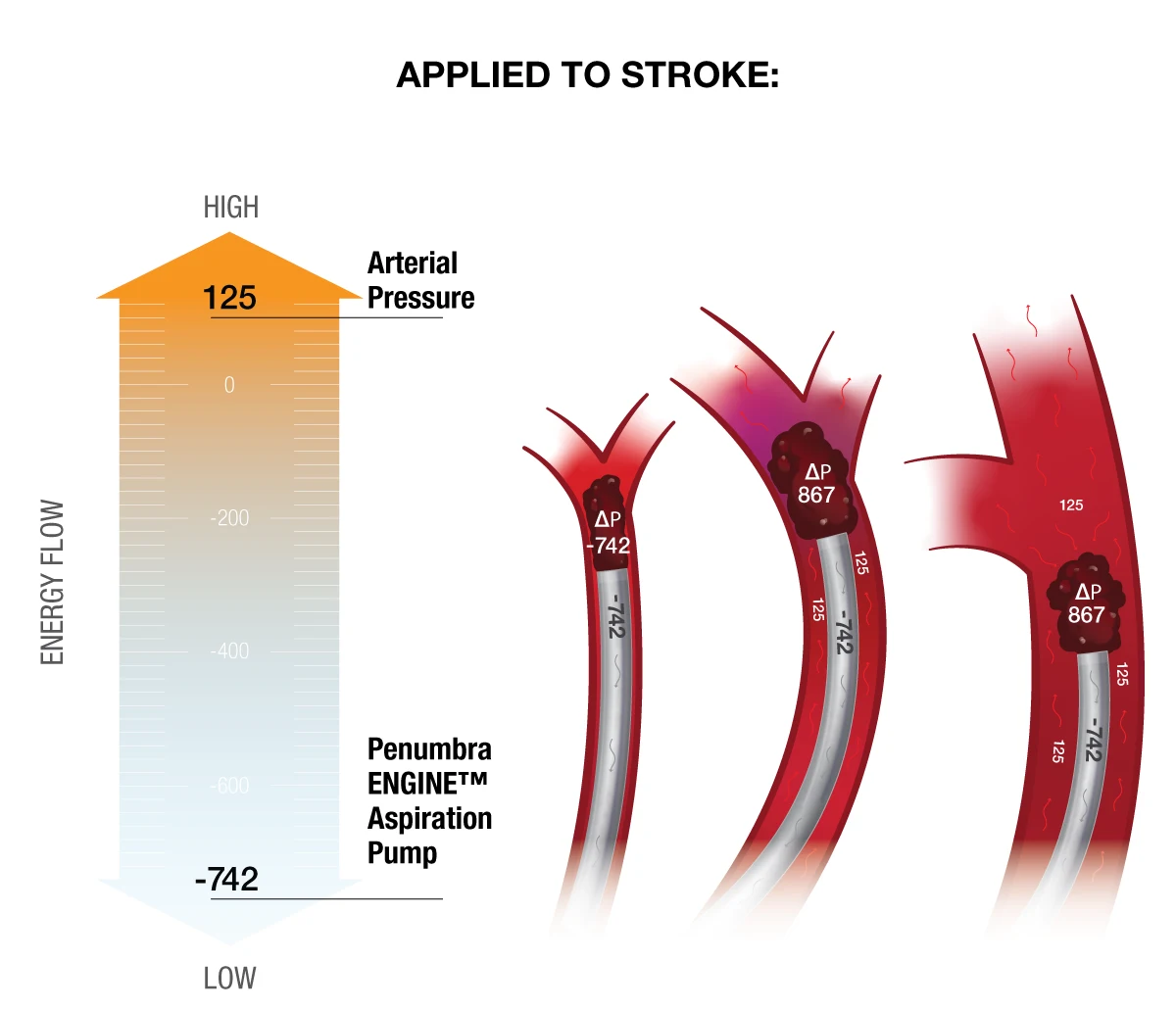

Figure 2: During an aspiration thrombectomy procedure, the clot and surrounding area is exposed to a low-pressure environment. The absolute difference between the systolic blood pressure and the vacuum is what pushes the clot into the canister.
2. Vessel Sizing:
Understanding average vessel sizing and the optimal catheter-to-vessel ratio for effective aspiration
Identifying the Optimal Catheter Size to Maximize Aspiration Efficiency
The understanding that exposure to systolic pressure is necessary for effective aspiration raises 2 key questions:
- What are the average cerebral vessel sizes?
- Which catheter diameters are appropriate in the various vessel segments?
True vessel sizing is a simple, albeit poorly understood, concept that lies at the heart of aspiration thrombectomy. Emerging research supports the theory that, on average, cerebral vessels are smaller than they are commonly believed to be. Additionally, the distribution of vessels in these studies demonstrates that Penumbra catheters are optimized for a broad group of patients.
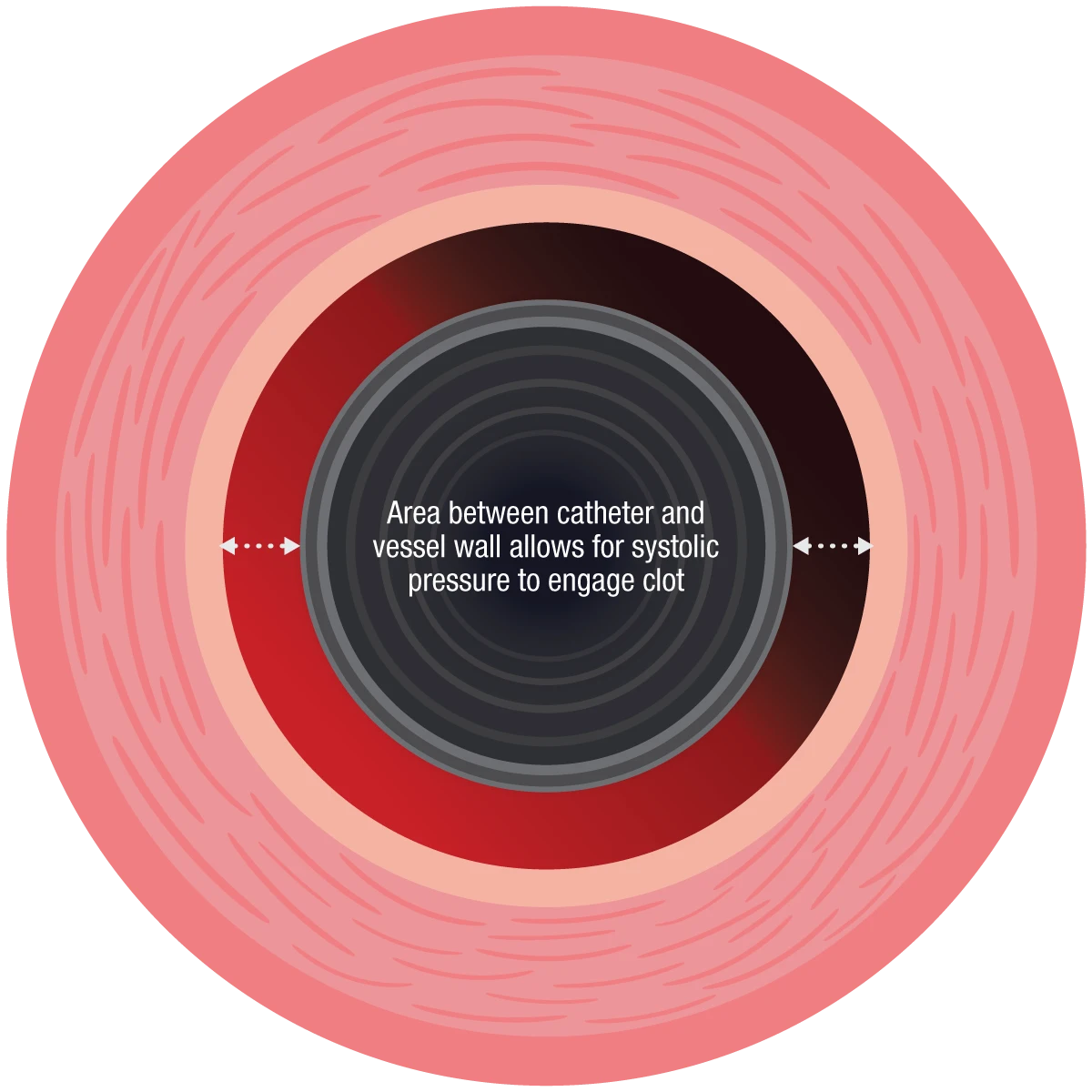

Figure 3: Area between catheter and vessel wall allows for systolic pressure to engage clot.
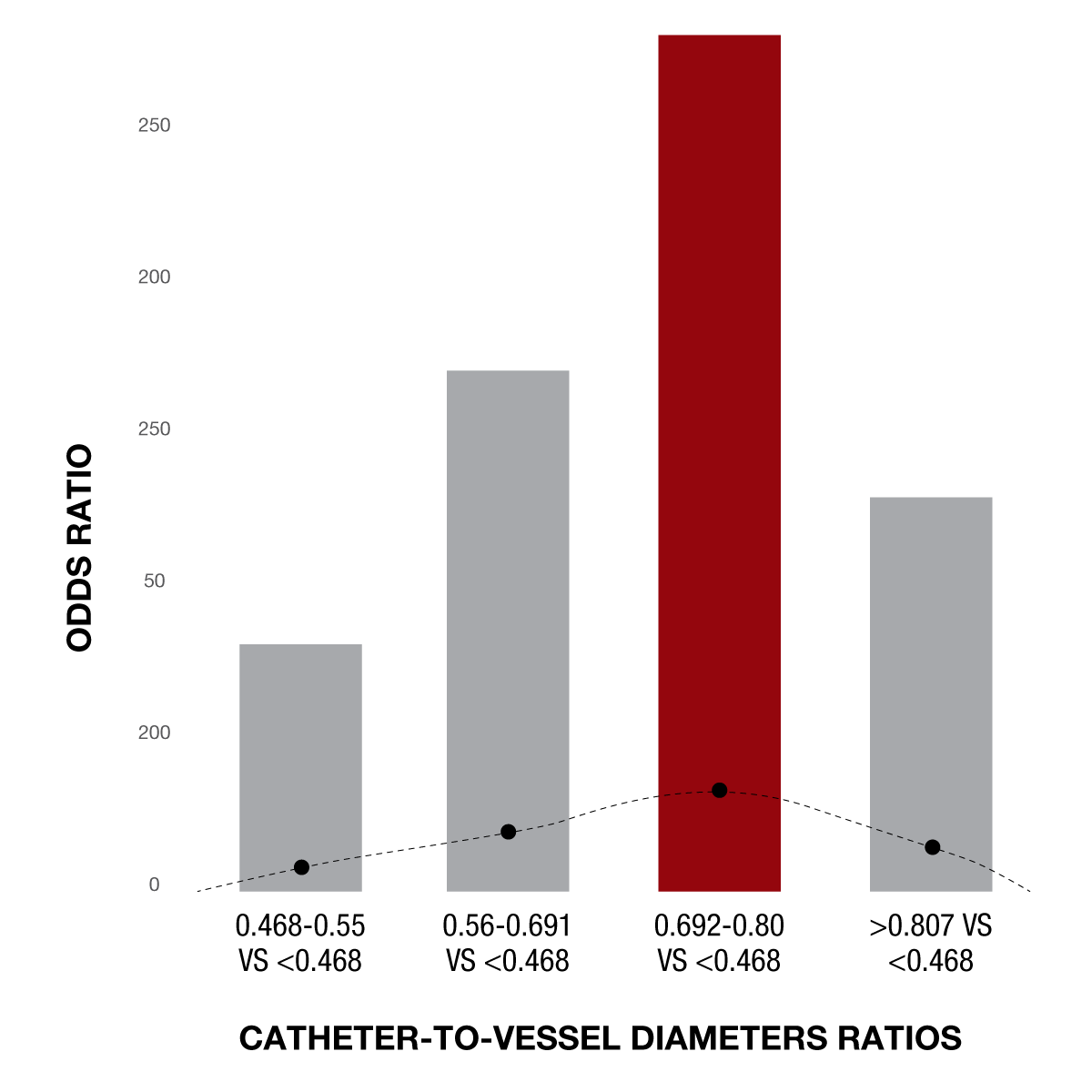

Figure 4: Recent data show that the optimal catheter ID-to-vessel diameter ratio is approximately 70-80%.1
What is the optimal catheter-to-vessel ratio?
As previously mentioned, the science of aspiration asserts that there are 2 variables that can be controlled during an aspiration thrombectomy procedure: the level of systolic pressure that is exposed to the low-pressure environment in the canister (Δ pressure differential), and the catheter lumen size (radius). In order to ensure these variables work together effectively, an appropriately sized catheter is essential.
Data suggests that the optimal ratio between catheter diameter and vessel wall is between 0.7 – 0.81 for the most favorable outcomes. Selecting a device above this range risks a reduction in the pressure differential necessary for aspiration. Conversely, selecting a device below this range risks a reduction in ingestion rates, ultimately slowing the procedure. Therefore, having a good working knowledge of the average vessel sizing in the neurovasculature and balancing the catheter-to-vessel ratio is a key factor in science-based aspiration thrombectomy.
1. Charbonnier G, Di Caterino F, Biondi A. Reply to “Successful recanalization because of the pressure differential between arterial systolic pressure (Push) and vacuum level created by the vacuum source (Pull)”. J Neuroradiol. 2024 Jun;51(4):101197. doi: 10.1016/j.neurad.2024.04.005. Epub 2024 Apr 25. PMID: 38664161.
What is the average vessel size?
According to recent clinical data from the STRATIS Registry (N = 665), the average M1 vessel is smaller than widely reported. Several other recent publications have also substantiated the theory that vessels may skew smaller than previously assumed. Since time is of the essence when a patient is suffering from a stroke, in an effort to prevent additional delays, many physicians do not measure vessels prior to treatment. By understanding the distribution of vessel diameters by segment across a population, physicians can make well-informed decisions when selecting a catheter to leverage systolic blood pressure during aspiration thrombectomy.
2. Saber H, et al. for the STRATIS Investigators. Variation in vessel size and angiographic outcomes following stent‐retriever thrombectomy in acute ischemic stroke: STRATIS Registry. STROKE Vasc Interv Neurol. 2024;4(3). doi:10.1161/SVIN.123.00097.
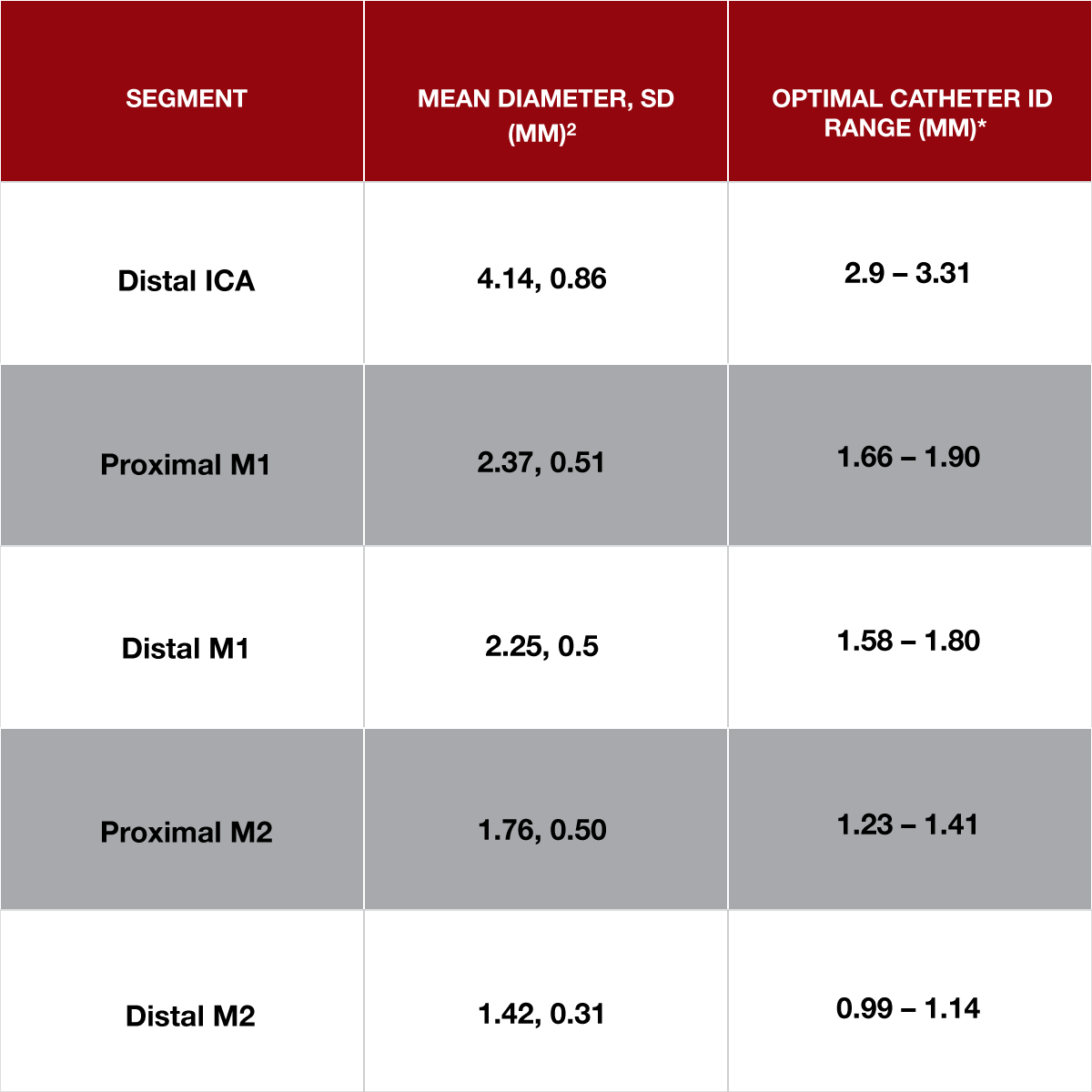

Figure 5: Data captured from the STRATIS Registry (N = 665) 2. *Mean diameters depicted used as a reference to calculate the optimal ID range based on the catheter ID-to-vessel diameter ratio shown in Figure 4.
3. Flow Control:
Evaluating the consequences of excessively restricting blood flow during a thrombectomy procedure
Consequences of Restricting Blood Flow During a Thrombectomy Procedure
Introducing a flow-limiting device into an artery does not come without risks. Flow control, or the practice of reducing flow to the target vessel and surrounding collaterals, has been shown to reduce the effectiveness of aspiration thrombectomy. Moreover, recent evidence suggests that flow control can hasten the conversion of penumbra to infarct, reducing the benefits of revascularization. Limiting the use of flow control when possible increases the probability of a successful thrombectomy and decreases stress on the delicate penumbra.
There are 2 primary methods to induce flow control during a thrombectomy procedure:
- Balloon-Guide Catheters: Designed to restrict flow in the ICA to prevent ENT
- High Placement of Super-Bore Catheters: Typically used for access to intracranial vessels with a secondary objective to reduce flow
While each method plays different roles in stroke care, they both ultimately work against the body’s natural systolic pressure, which can result in unfavorable effects during aspiration thrombectomy.
3. Requena M, Li J, Tiberi R, et al. Impact on collateral flow of devices used for endovascular treatment of stroke: an in-vitro flow model. J Neurointerv Surg. Published online August 30, 2023:jnis-2023-020602. doi:10.1136/jnis-2023-020602.
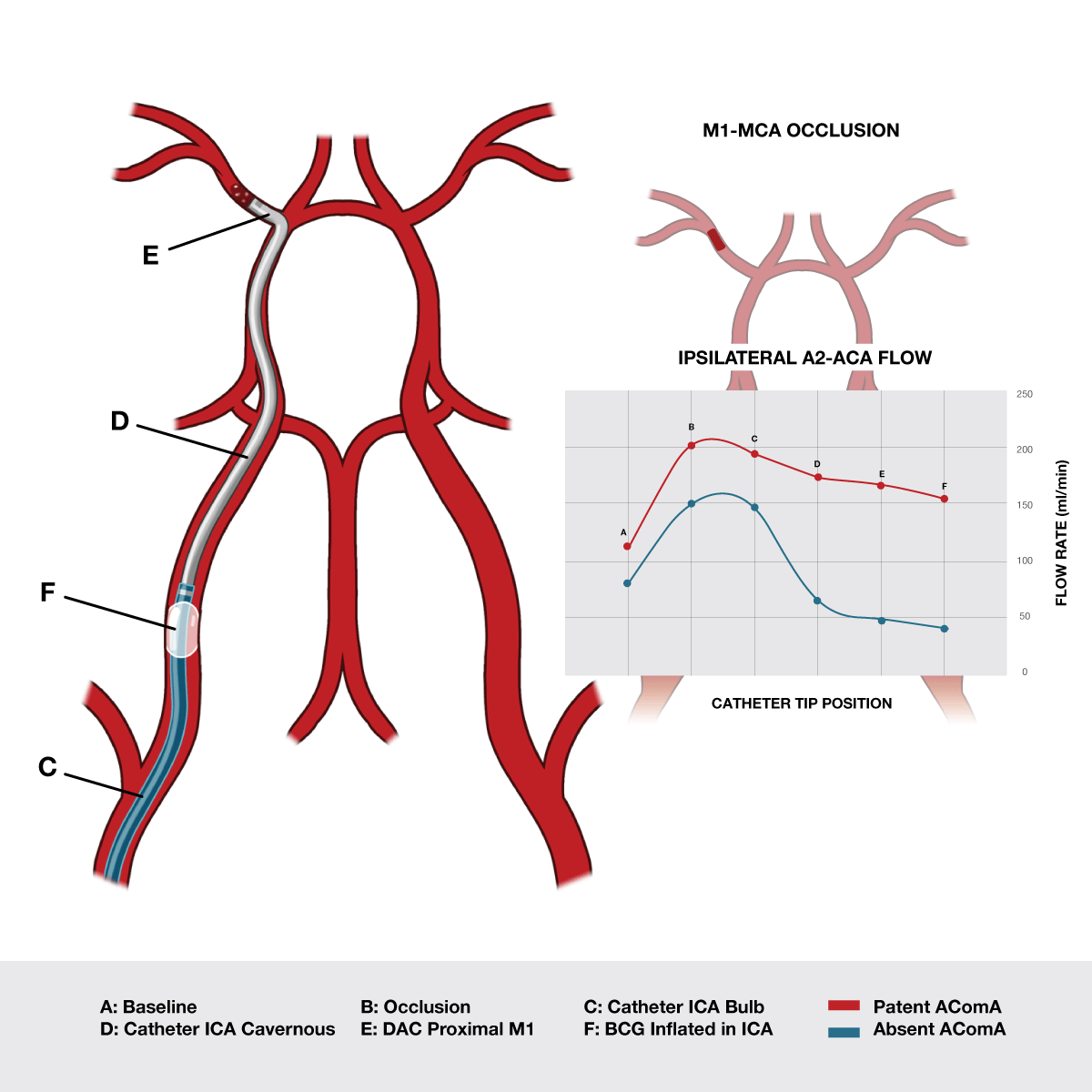

Figure 6: Data from a recent in-vitro study published in JNIS show that collateral flow decreases by as much as 74% as devices progressively occupy the intravascular space.3
4. Time:
Analyzing procedure time in relation to patient outcomes, and key opportunities to reduce time
The Effect of Procedure Time and Efficiency on Long-Term Outcomes
In stroke care, time is brain. It is widely known and accepted that the faster a clot is removed, the more favorable patient outcomes can be. However, there is now evidence that procedure efficiency is a significant piece of the equation. Time and procedure efficiency are not mutually exclusive.
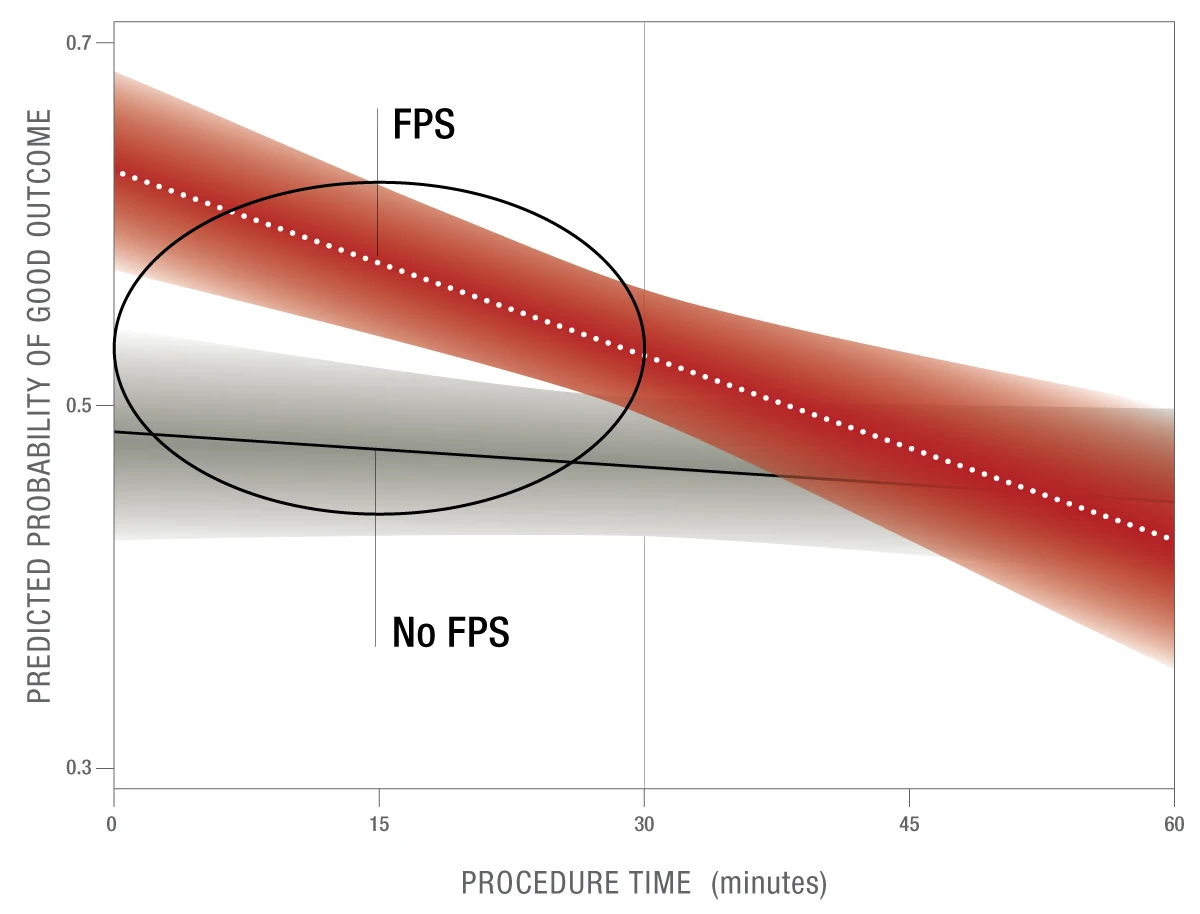

Figure 7: FPE30 is a proposed modification to the current FPE procedural benchmark that explores the impact of procedure time on a first pass success.
A first pass success in < 30 minutes results in 11.5% higher probability of good outcomes than those that required > 2 passes4
These results were statistically significant.
Introducing First Pass Effect 30 (FPE30)
A retrospective analysis from the STAR Registry, a multi-center international dataset, assessed 1,310 LVO-AIS patients that had achieved excellent reperfusion (mTICI 2c/3). The primary study exposure was a first pass success and the secondary study exposure was procedure time.
Patients showed no incremental benefit from a first pass success if completed in > 30 minutes. This data emphasizes the importance of standardized stroke algorithms and an understanding of the science behind aspiration in order to leverage the body’s natural processes to aid in efficient aspiration thrombectomy.
4. Koo AB, Reeves BC, Renedo D, Maier IL, et al. Impact of procedure time on first pass effect in mechanical thrombectomy for anterior circulation acute ischemic stroke. Neurosurg. 2024;95(1):128-136. doi: 10.1227/neu.0000000000002900.
Want to Learn More?
Contact us to learn more about S-BAT and schedule a demo
