Penumbra Announces First Patient Enrolled in Study of Mechanical Power Aspiration for Thrombus Removal in Coronary Vessels
Prospective Study to Evaluate Safety and Performance of Indigo® System and CAT™ RX Aspiration Catheter
ALAMEDA, Calif. –
Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, today announced enrollment of the first patient into the CHEETAH study, a prospective, multi-center U.S. study to evaluate the safety and performance of the Indigo System with CAT RX Aspiration Catheter in the coronary vessels. Introduced in 2018, as part of the Indigo Aspiration System, the Indigo CAT RX Aspiration Catheters and Indigo Separator™ 4 are indicated for the removal of fresh, soft emboli and thrombi from vessels in the coronary and peripheral vasculature.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191010005238/en/
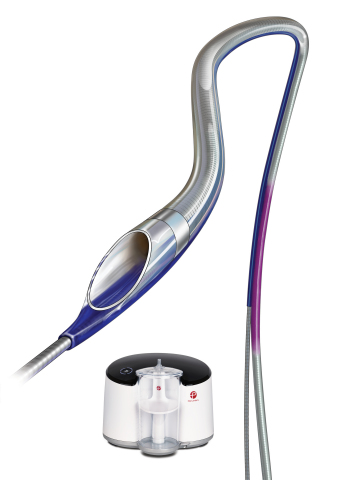
Indigo® System CAT™ RX Aspiration Catheter and Penumbra ENGINE™. As part of the Indigo Aspiration System, the Indigo CAT RX Aspiration Catheters and Indigo Separator™ 4 are indicated for the removal of fresh, soft emboli and thrombi from vessels in the coronary and peripheral vasculature. (Photo: Business Wire)
“This important study will help inform us of the potential impact of clot removal in patients with high thrombus burden,” said Larry J. Diaz-Sandoval, M.D., director, endovascular research and interventions, Metro Health-University of Michigan Health Hospital, Grand Rapids, Michigan, whose team enrolled the first patient in the CHEETAH study.
The Indigo System CAT RX device uses mechanical power aspiration to remove thrombus in the coronaries. The post-market, prospective CHEETAH study will enroll up to 400 patients presenting with coronary thrombus who are referred for percutaneous coronary intervention (PCI) at up to 25 U.S. centers (ClinicalTrials.gov Identifier: NCT03957473). The primary study endpoint is a composition of cardiovascular (CV) death, recurrent myocardial infarction (MI), cardiogenic shock or new or worsening New York Heart Association (NYHA) Class IV heart failure within 30 days. Secondary endpoints include final TIMI flow grade, final TIMI thrombus grade, and safety assessments at six months.
“Penumbra has adapted over 10 years of neuro thrombectomy experience to address the limitations of traditional manual aspiration for the coronaries by development of the Indigo System CAT RX device,” said national principal investigator S. Jay Mathews, M.D., director, cardiac catheterization laboratory, Manatee Memorial Hospital in Bradenton, Florida. “This significant upgrade in innovation from syringe-based aspiration to mechanical power aspiration coupled with highly trackable catheter technology has enabled us to improve our door to reperfusion times and thereby to improve patient care.”
“Just like the IMS-III trial for ischemic stroke, the TOTAL trial highlighted that new therapies are needed to improve the outcomes of these patients with high thrombus burden,” said Jasvindar Singh, M.D., associate professor of medicine and director of cardiac catheterization laboratory at Barnes Jewish Hospital/ Washington University School of Medicine, St. Louis, Missouri. “The CHEETAH study is the first step toward making coronary mechanical thrombectomy standard of care for high thrombus burden patients. We expect this study to refine our technique of thrombus aspiration and better understand the utilization of CAT RX for patients with high thrombus burden.”
About the Indigo System
In 2014, Penumbra introduced the Indigo System, a continuous aspiration mechanical thrombectomy system designed to remove clot from arteries and veins in the peripheral vasculature. The Indigo System utilizes the Penumbra ENGINE™ aspiration source to deliver nearly pure, continuous vacuum suction to the Indigo System Aspiration Catheters to address thrombus in vessels of various sizes. The Indigo System CAT RX Aspiration Catheter was introduced in 2018. As part of the Indigo System, the Indigo CAT RX Aspiration Catheters and Indigo Separator 4 are indicated for the removal of fresh, soft emboli and thrombi from vessels in the coronary and peripheral vasculature. The Indigo CAT RX is a 6-French compatible, rapid exchange catheter designed for maximized clot removal with its large aspiration lumen and engineered for advanced trackability and deliverability as a result of seven material transitions and a proximal laser cut hypotube. The proprietary Separator 4 may be used together with the Indigo CAT RX for mechanical clot engagement.
Important Safety Information
Additional information about Penumbra’s products can be located on Penumbra’s website at http://www.penumbrainc.com/healthcare-professionals. Prior to use, please refer to Instructions for Use for complete product indications, contraindications, warnings, precautions, potential adverse events and detailed instructions for use.
About Penumbra
Penumbra, Inc., headquartered in Alameda, Calif., is a global healthcare company focused on innovative therapies. Penumbra designs, develops, manufactures and markets innovative products and has a broad portfolio that addresses challenging medical conditions and significant clinical needs across two major markets, neuro and vascular. Penumbra sells its products to hospitals primarily through its direct sales organization in the United States, most of Europe, Canada and Australia, and through distributors in select international markets. Penumbra, the Penumbra P logo, Indigo, CAT, Separator and Penumbra ENGINE are trademarks of Penumbra, Inc. For more information, visit www.penumbrainc.com.
Forward-Looking Statements
Except for historical information, certain statements in this press release are forward-looking in nature and are subject to risks, uncertainties and assumptions about us. Our business and operations are subject to a variety of risks and uncertainties and, consequently, actual results may differ materially from those projected by any forward-looking statements. Factors that could cause actual results to differ from those projected include, but are not limited to: failure to sustain or grow profitability or generate positive cash flows; failure to effectively introduce and market new products; delays in product introductions; significant competition; inability to further penetrate our current customer base, expand our user base and increase the frequency of use of our products by our customers; inability to achieve or maintain satisfactory pricing and margins; manufacturing difficulties; permanent write-downs or write-offs of our inventory; product defects or failures; unfavorable outcomes in clinical trials; inability to maintain our culture as we grow; fluctuations in foreign currency exchange rates; and potential adverse regulatory actions. These risks and uncertainties, as well as others, are discussed in greater detail in our filings with the Securities and Exchange Commission, including our Quarterly Reports on Form 10-Q and our Annual Report on Form 10-K for the year ended December 31, 2018. There may be additional risks of which we are not presently aware or that we currently believe are immaterial which could have an adverse impact on our business. Any forward-looking statements are based on our current expectations, estimates and assumptions regarding future events and are applicable only as of the dates of such statements. We make no commitment to revise or update any forward-looking statements in order to reflect events or circumstances that may change.
Source: Penumbra, Inc.
View source version on businesswire.com: https://www.businesswire.com/news/home/20191010005238/en/
MEDIA CONTACT:
Betsy Merryman
Merryman Communications
[email protected]
310-560-8176
