Penumbra Launches Newest Stroke Thrombectomy Technology ACE™68 Reperfusion Catheter at SNIS 2016
Clinical Experience of Latest Stroke Revascularization Solution to be
Highlighted in Symposium at Meeting
BOSTON –
Penumbra,
Inc. (NYSE:PEN), a global interventional therapies company, today
announced U.S. commercial availability of its most advanced thrombectomy
device, the ACE™68 Reperfusion Catheter, part of the fully integrated
Penumbra System®, at the Society of NeuroInterventional
Surgery (SNIS) 13th Annual Meeting in Boston, Massachusetts.
This Smart News Release features multimedia. View the full release here:
http://www.businesswire.com/news/home/20160725005231/en/
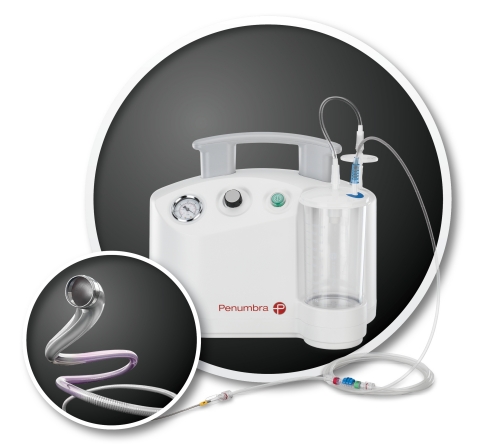
The ACE™68 Reperfusion Catheter, part of the fully integrated Penumbra System®, leverages the latest advancements in tracking technology to deliver maximum aspiration power easily and safely for extracting thrombus in acute ischemic stroke patients. (Photo: Business Wire)
The ACE68 Reperfusion Catheter leverages the latest advancements in
tracking technology to deliver maximum aspiration power easily and
safely for extracting thrombus in acute ischemic stroke patients.
Clinical experience with the ACE68 will be shared today from 1:30-1:45
p.m. ET in the Industry Technology Luncheon Symposium by Blaise Baxter,
M.D., chief of radiology at Erlanger Hospital in Tennessee and chairman
of radiology for the University of Tennessee College of Medicine
Chattanooga.
“The tracking technology of the ACE68 Reperfusion Catheter is the most
advanced,” said Baxter. “In my clinical experience with the ACE68, I saw
the device easily navigate difficult tortuosity that would have
challenged other devices. ACE68’s tracking performance, combined with a
large aspiration lumen to enable efficient clot removal, make ACE68 the
most compelling frontline device in stroke intervention.”
The ACE68 Reperfusion Catheter was engineered on a new, innovative
tracking platform from hub to tip. Featuring a unique coil winding
geometry along 16 transitions to create the optimal tracking profile,
ACE68 is designed to ensure easy tracking through tortuosity that is
typical in acute ischemic stroke patients. ACE68 is powered to extract
clot en masse quickly and effectively as part of the fully integrated
Penumbra System.
“With the ACE68 Reperfusion Catheter, I can easily deliver full
aspiration power to the occlusion,” said Johanna Fifi, M.D., assistant
professor of neurology, neurosurgery and radiology at The Mount Sinai
Hospital and director of the Endovascular Stroke Program at the Mount
Sinai Health System in New York. “The ACE68’s large lumen increases the
likelihood of capturing the clot fully within the catheter or the
canister, potentially reducing the number of passes to achieve complete
revascularization and minimize ENT (embolization to new territory).”
“The ACE68 provides an opportunity to reverse strokes faster and with
less expense,” said Adam Arthur, M.D., MPH, FACS, professor, Department
of Neurosurgery, UTHSC, Semmes-Murphey Neurologic & Spine Institute.
“The larger lumen seems to allow better clot capture, which may reduce
the need for adjunctive devices, simplify the procedure and reduce
procedure cost — important considerations as hospitals look to expand
stroke services.”
The ACE68 represents the latest advances in tracking technology to
deliver a large bore reperfusion catheter easily and reliably through
tortuosity that is typical in acute ischemic stroke patients.
“We designed the ACE68 with the intent to make real improvement on
stroke procedure time, outcome and cost. The early reports from
physicians on the performance of ACE68 confirm that this is the most
impactful stroke product we have ever developed,” said Adam Elsesser,
chairman and chief executive officer of Penumbra.
About the ACE68 Reperfusion Catheter and the Penumbra System
The Penumbra System with the ACE68 Reperfusion Catheter is indicated for
use in the revascularization of patients with acute ischemic stroke
secondary to intracranial large vessel occlusive disease within eight
hours of symptom onset. The Penumbra System consists of large, highly
pliable and trackable Reperfusion Catheters that utilize the full
aspiration power of the Penumbra Pump MAX™ within an integrated,
proprietary system to remove the stroke-causing occlusion safely and
effectively. The Penumbra System offers a complete suite of reperfusion
catheters sized optimally for large vessel occlusions.
The Penumbra System with the ACE68 Reperfusion Catheter is now available
in the U.S.
About Penumbra
Penumbra, Inc., headquartered in Alameda, California, is a global
interventional therapies company that designs, develops, manufactures
and markets innovative medical devices. The company has a broad
portfolio of products that address challenging medical conditions and
significant clinical needs across two major markets, neuro and
peripheral vascular. Penumbra sells its products to hospitals primarily
through its direct sales organization in the U.S., most of Europe,
Canada and Australia, and through distributors in select international
markets. Penumbra and the Penumbra logo are trademarks of Penumbra, Inc.
Forward-Looking Statements
Except for historical information, certain statements in this press
release are forward-looking in nature and are subject to risks,
uncertainties and assumptions about us. Our business and operations are
subject to a variety of risks and uncertainties and, consequently,
actual results may differ materially from those projected by any
forward-looking statements. Factors that could cause actual results to
differ from those projected include, but are not limited to: failure to
sustain or grow profitability or generate positive cash flows; failure
to effectively introduce and market new products; delays in product
introductions; significant competition; inability to further penetrate
our current customer base, expand our user base and increase the
frequency of use of our products by our customers; inability to achieve
or maintain satisfactory pricing and margins; manufacturing
difficulties; permanent write-downs or write-offs of our inventory;
product defects or failures; unfavorable outcomes in clinical trials;
inability to maintain our culture as we grow; fluctuations in foreign
currency exchange rates; and potential adverse regulatory actions. These
risks and uncertainties, as well as others, are discussed in greater
detail in our filings with the Securities and Exchange Commission,
including our Quarterly Reports on Form 10-Q and our Annual Report on
Form 10-K for the year ended December 31, 2015. There may be additional
risks of which we are not presently aware or that we currently believe
are immaterial which could have an adverse impact on our business. Any
forward-looking statements are based on our current expectations,
estimates and assumptions regarding future events and are applicable
only as of the dates of such statements. We make no commitment to revise
or update any forward-looking statements in order to reflect events or
circumstances that may change.
Source: Penumbra, Inc.
View source version on businesswire.com: http://www.businesswire.com/news/home/20160725005231/en/
Merryman Communications
Betsy Merryman
310-560-8176
[email protected]
