Penumbra Augments Vascular Franchise with Latest Indigo System Launch and Expands Medical/Scientific Leadership
Launches Indigo® System Lightning™ 12 in U.S.
Appoints Corey L. Teigen, M.D., as Chief Scientific Officer and James F. Benenati, M.D., FSIR, as Chief Medical Officer
ALAMEDA, Calif. –
Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, today announced its next phase for vascular franchise growth with U.S. commercial availability of the Indigo® System Lightning™ 12 and the appointments of Corey L. Teigen, M.D., to chief scientific officer and James F. Benenati, M.D., FSIR, to chief medical officer, two newly created positions. Innovating the Indigo System peripheral thrombectomy technology is a key part of the company’s overall growth plan to continue momentum in its fast-growing vascular franchise.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200714005400/en/
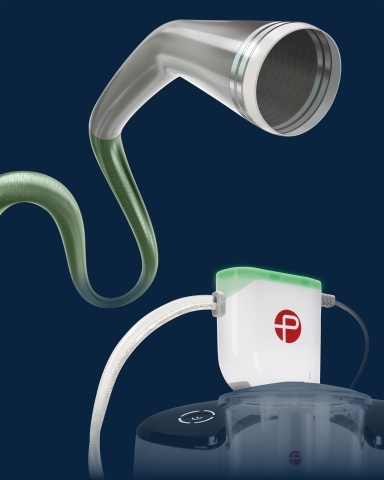
Lightning™ 12 – Intelligent Aspiration Powered by Penumbra ENGINE™ (Photo: Business Wire)
Indigo System Lighting 12: The Most Advanced Clot Removal Technology
The Indigo System Lightning 12 is the company’s next generation aspiration system for peripheral thrombectomy. Lightning 12 combines the new Indigo System CAT™12 Aspiration Catheter with Lightning™ Intelligent Aspiration, enabling physicians to focus on optimizing thrombus removal using the system’s unique clot detection mechanism. CAT12 is a large-lumen aspiration catheter that incorporates novel laser-cut hypotube-based catheter engineering to provide advanced deliverability and torqueability within the body. This combination of intelligent aspiration and large-lumen catheter engineering makes Lightning 12 Penumbra’s most advanced clot removal technology.
“Penumbra continues to lead the field of clot management by bringing highly innovative technology to address the challenges that we as physicians face while caring for our patients,” said Frank Arko, M.D., chief, Division of Vascular and Endovascular Surgery, Sanger Heart and Vascular Institute, North Carolina. “When dealing with thrombus, we have learned that it is the combination of the catheter along with powered aspiration that yields the most effective results. Lightning 12 with Intelligent Aspiration is a giant leap forward for the field of thrombectomy, and we have been very impressed with the early results at Sanger.”
“The simplicity of Lightning Intelligent Aspiration combined with the significant advancements in catheter engineering will enable us as physicians to get closer to our thrombus removal goal in a safe manner, as well as our goal of improving clinical outcomes for our patients,” said Patrick Muck, M.D., chief, Vascular Surgery, Good Samaritan Hospital, Ohio.
“Lightning 12 provides physicians with an integrated system that not only removes large amounts of thrombus but also detects and manages clot removal,” said Jay Mathews, M.D., interventional cardiologist, Manatee Memorial Hospital, Florida. “This is a very important advancement for the field of thrombus management, and our initial experience at Manatee Memorial with this technology shows us that we are now closer to single-setting care for our patients.”
New Leadership Appointments
Penumbra also announced appointments for two newly created positions: Corey L. Teigen, M.D., as chief scientific officer and James F. Benenati, M.D., FSIR, as chief medical officer.
Dr. Teigen joins immediately as CSO and will contribute his extensive scientific and clinical expertise to ongoing and future research and development efforts.
“In my radiology practice, I’ve seen firsthand the challenges medical practitioners face while treating difficult medical conditions,” Dr. Teigen said. “At Penumbra, I have a profound opportunity to change the course of healthcare delivery for people living with vascular disease. I feel privileged to join Penumbra’s unparalleled group of talented engineers and leaders, and I look forward to contributing my scientific knowledge to help solve challenging medical problems.”
Dr. Benenati will join Penumbra on September 1, 2020, as CMO, and will contribute to clinical and medical affairs strategies and advise upon global commercialization and market development activities.
“I’m excited to join Penumbra at this very important time for the company, where it’s poised for growth, as it moves into the next generation of intelligent clot removal and expand into rehabilitation with the recent launch of the REAL system,” Dr. Benenati said. “Penumbra has changed the landscape of healthcare by providing truly innovative solutions for a wide realm of medical challenges. In this next phase, Penumbra is set to broaden its reach on a global scale, and I am thrilled to join this excellent team and help accelerate the impact of its products.”
“I am thrilled we are bringing Lightning 12, the most advanced technology for aspiration thrombectomy, to the peripheral interventional community,” said Adam Elsesser, president and chief executive officer, Penumbra. “It is an ideal time for us to welcome Corey and Jim to Penumbra’s leadership team as we progress into our next phase of growth. Their career-long focus on patient care and their innovative mindset are a perfect fit with our vision and culture, which is dedicated to making a fundamental difference in patients’ lives.”
About Corey L. Teigen, M.D.
Corey L. Teigen, M.D., is a board certified vascular and interventional radiologist. He is also a researcher and developer of interventional devices. Dr. Teigen received his medical degree from Johns Hopkins University School of Medicine with distinction and did his radiology residency at the Mayo Clinic Rochester, Minnesota. He completed his fellowship at the Miami Cardiac and Vascular Institute, Miami, Florida. He served as Chairman of Interventional Radiology at Sanford Health, Fargo, North Dakota for 25 years. Dr. Teigen has authored numerous publications and has been involved in the development of multiple interventional devices for the treatment of vascular and other diseases. He has presented more than 100 professional talks sharing lessons learned and successes realized over his 30-year career in the practice of Interventional Radiology. Dr. Teigen’s research has focused on multiple areas including development of the Cordis Incraft stent graft for the endovascular repair of abdominal aneurysm. He has also been involved in the development of intravascular stents, balloons, liquid and coil embolics, inferior vena cava filters, and thrombectomy devices. He has served on the Peripheral Vascular Committee of SIR and was Co-director of the Sanford Health Vascular Institute.
About James F. Benenati, M.D., FSIR
James F. Benenati, M.D., FSIR, is a board certified vascular and interventional radiologist. Prior to joining Penumbra, Dr. Benenati practiced interventional radiology at the Miami Cardiac & Vascular Institute at Baptist Hospital in Miami for 30 years, where he has served in multiple positions, including medical director of the noninvasive vascular laboratory and fellowship program director. He is the vice chairman of the department of interventional radiology and clinical professor of radiology at the Florida International University Herbert Wertheim College of Medicine, Miami. He is also a Collaborative Professor of Radiology at the University of South Florida Morsani College of Medicine. He is past President of the Society of Interventional Radiology (SIR 2011) and past Annual Meeting Chairman for the SIR Annual Meeting in 2005. He has received the highest awards given by the SIR including the SIR Gold Medal and the Charles T. Dotter Award. Dr. Benenati is the founding Medical Director of the Nova Southeastern University Health Sciences BS/ RVT program. He served as President of the Vascular Division for the Intersocietal Accreditation Committee (IAC) and has served for over a decade on the IAC Vascular Testing Board. Over the course of his career, Dr. Benenati has authored more than 120 scientific publications, published numerous book chapters and edited journals and textbooks in interventional radiology. He has lectured nationally and internationally, and has been a program director of the International Symposium on Endovascular Technology (ISET) for 30 years. His undergraduate education was at the University of Notre Dame, he attended medical school at the University of South Florida College of Medicine from 1980-1984, he completed a diagnostic radiology residency at Indiana University School of Medicine in 1988, and he completed a fellowship in Cardiovascular and Interventional Radiology at the Johns Hopkins University School of Medicine in 1989.
About Indigo System
The latest generation of Penumbra’s continuous aspiration thrombectomy system features Indigo System Lightning 12 which combines the new Indigo System CAT 12 Aspiration Catheter with Lightning Intelligent Aspiration powered by Penumbra ENGINE, enabling physicians to focus on optimizing thrombus removal using the system’s unique clot detection mechanism. The Indigo System’s proprietary Separator technology, Separator 12 is also available with Lightning 12 and is designed to enable unobstructed aspiration for the duration of the procedure. Lightning 12 and Separator 12 are designed for the removal of fresh, soft emboli and thrombi from the peripheral arterial and venous systems.
In addition to Lightning 12, the Indigo System also now includes Lightning 8 which combines the Indigo System CAT 8 Aspiration Catheter with Lightning Intelligent Aspiration for the removal of fresh, soft emboli and thrombi from the peripheral arterial and venous systems, and for the treatment of pulmonary embolism. Lightning 12 and Lightning 8 expand the already broad offering of CAT8, CATD, CAT6, CAT5, and CAT3 and are paired with Penumbra ENGINE, the company’s proprietary continuous, mechanical vacuum aspiration pump.
Important Safety Information
Additional information about Penumbra’s products can be located on Penumbra’s website at http://www.penumbrainc.com/healthcare-professionals. Prior to use, please refer to Instructions for Use for complete product indications, contraindications, warnings, precautions, potential adverse events and detailed instructions for use.
About Penumbra
Penumbra, Inc., headquartered in Alameda, California, is a global healthcare company focused on innovative therapies. Penumbra designs, develops, manufactures and markets novel products and has a broad portfolio that addresses challenging medical conditions in markets with significant unmet need. Penumbra sells its products to hospitals and healthcare providers primarily through its direct sales organization in the U.S., most of Europe, Canada and Australia, and through distributors in select international markets. Penumbra, the Penumbra P logo, Indigo, CAT, Separator, Lightning, Penumbra ENGINE and REAL are trademarks of Penumbra, Inc. For more information, visit www.penumbrainc.com and connect on Twitter and LinkedIn.
Forward-Looking Statements
Except for historical information, certain statements in this press release are forward-looking in nature and are subject to risks, uncertainties and assumptions about us. Our business and operations are subject to a variety of risks and uncertainties and, consequently, actual results may differ materially from those projected by any forward-looking statements. Factors that could cause actual results to differ from those projected include, but are not limited to: the impact of the COVID-19 pandemic on our business, results of operations and financial condition; failure to sustain or grow profitability or generate positive cash flows; failure to effectively introduce and market new products; delays in product introductions; significant competition; inability to further penetrate our current customer base, expand our user base and increase the frequency of use of our products by our customers; inability to achieve or maintain satisfactory pricing and margins; manufacturing difficulties; permanent write-downs or write-offs of our inventory; product defects or failures; unfavorable outcomes in clinical trials; inability to maintain our culture as we grow; fluctuations in foreign currency exchange rates; and potential adverse regulatory actions. These risks and uncertainties, as well as others, are discussed in greater detail in our filings with the Securities and Exchange Commission, including our Annual Report on Form 10-K for the year ended December 31, 2019 filed with the SEC on February 26, 2020, and our Quarterly Reports on Form 10-Q. There may be additional risks of which we are not presently aware or that we currently believe are immaterial which could have an adverse impact on our business. Any forward-looking statements are based on our current expectations, estimates and assumptions regarding future events and are applicable only as of the dates of such statements. We make no commitment to revise or update any forward-looking statements in order to reflect events or circumstances that may change.
Source: Penumbra, Inc.
View source version on businesswire.com: https://www.businesswire.com/news/home/20200714005400/en/
Investor Relations:
Penumbra, Inc.
[email protected]
510-995-2461
Media Relations:
Joni Ramirez
Merryman Communications
[email protected]
323-532-0746
