Penumbra Announces CE Mark for the ELEMENT Vascular Access System and the Select + Catheter, Completing Its VTE Thrombectomy Platform
Penumbra Inc., the world’s leading thrombectomy company, has secured CE Mark and launched the ELEMENT™ Vascular Access System, the first laser-cut hypotube sheath designed for venous thromboembolism (VTE). The ELEMENT Vascular Access System is compatible with Lightning Flash™ 2.0, the most powerful and advanced mechanical thrombectomy system for the removal of venous thrombus and the treatment of pulmonary embolism (PE).
The company also announced a CE Mark for its Select™ + Catheter, effectively completing Penumbra’s VTE thrombectomy platform, from access to aspiration. Select + is engineered for the pulmonary anatomy, creating a seamless transition and designed for safe access through the heart.


Above: Select + Catheter H1 tip shape
“The flexibility and torqueability of the Lightning Flash 2.0 catheter paired with the foundational support of the ELEMENT sheath has allowed us excellent angiographic and clinical success,” said Raj Kakarla, M.D., interventional radiologist at Javon Bea Hospital in Rockford, Illinois. “Penumbra’s continuous advancements provide a comprehensive portfolio for physicians to treat a wider range of patients and improve patient outcomes.”
Innovative Valve Technology with the HemoLock™ Dual Valve System
ELEMENT features the HemoLock™ Valve System, designed to ensure hemostasis through dual-valve engineering. Equipped with a rotating hemostatic valve and a removable cross-cut valve, the system provides physicians with precise control during procedures.
Advanced Hypotube Engineering — Optimized for Enhanced Delivery and Performance
ELEMENT has a laser-cut hypotube design that was engineered to maintain stepwise support throughout the vasculature to enhance performance across challenging anatomies. The atraumatic tip design is meticulously crafted to help enable smooth delivery and trackability. The proximal segment is purposefully engineered to deliver foundational support. Optimized polymer transitions help maintain sheath access without the application of proximal hydrophilic coatings.
The ELEMENT hypotube is composed of 17 French MaxID technology, tailor-made for use in conjunction with the Lightning Flash 2.0, CAT™16 catheter. This technology enables in-case visualization via hand injection around the CAT16 and provides sufficient space to torque CAT16.
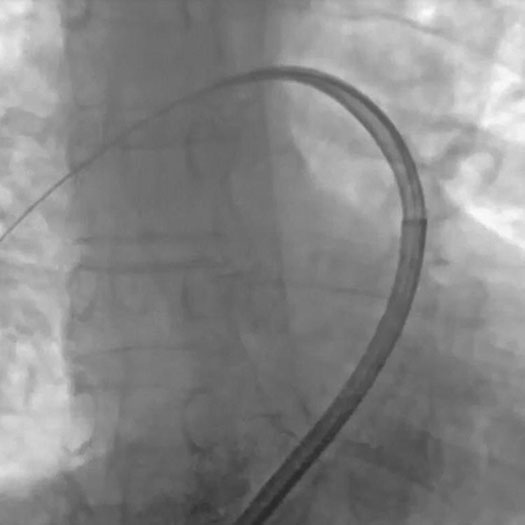

ELEMENT tracking with Dilator | Dr. Raj Kakarla, Mercy Health Javon Bea, Rockford, IL
Important Safety Information
Additional information about Penumbra’s products can be located on Penumbra’s website at https://www.penumbrainc.com/eu/products/peripheral-thrombectomy-indigo-system/. Product availability varies by country. Prior to use, please refer to Instructions for Use for complete product indications, contraindications, warnings, precautions, potential adverse events and detailed instructions for use. Risk information can be found at peninc.info/risk.
Copyright ©2025 Penumbra, Inc. All rights reserved. ELEMENT, Select +, Lightning Flash, HemoLock, and CAT are registered trademarks or trademarks of Penumbra, Inc. in the USA and other countries.
Related Articles
-
Penumbra Receives CE Mark for MIDWAY and ACCESS25 Delivery Catheters in Europe
December 17, 2025 -
Penumbra Receives CE Mark for Lightning Bolt 12 and Lightning Bolt 6X with TraX in Europe
December 2, 2025 -
New COMPLETE Registry Findings Offer Important Data on Vessel Size for Device Choice
September 29, 2025 -
Endovascular Today: How Science-Based Aspiration Principles Inform Effective Stroke Care
September 26, 2025
