RED™ 43 Reperfusion Catheter
Designed for Smoother Delivery and Increased Distal Aspiration
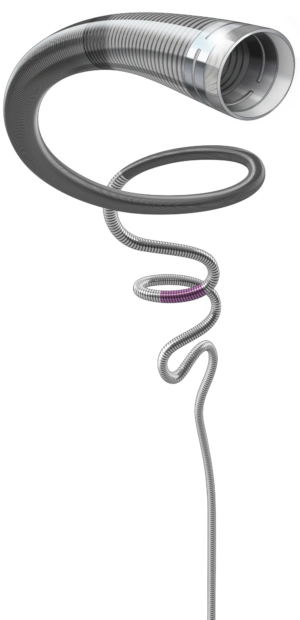

- 1.09 mm (.043″) Lumen: Delivers powerful distal aspiration
- 1.52 mm (.060″) Outer Diameter: Low profile and atraumatic design for distal deliverability
- Compatible with RED 62, RED 68, and RED 72: Optimised lumen occupation
- REDglide Technology: Designed to enhance delivery in tortuosity
- Precision Tip: Articulating marker band with short polymer tip design for optimal visualisation and placement
- Full-Length PTFE Liner: Designed for durability and lumen integrity under powerful aspiration
- Proximal Hybrid Stainless Steel & Nitinol Coil Wind: Engineered to provide optimal support
- 160 cm Length: To reach target vessel
For Australian health professionals only. RED 43, RED 62, RED 68, and RED 72 are not available for general supply in Australia, they are only available through the Special Access Scheme.
Prior to use, please refer to the Instructions for Use for complete product indications, contraindications, warnings, precautions, potential adverse events, and detailed instructions for use.
PENUMBRA SYSTEM – Intended Use
The PENUMBRA SYSTEM is intended for use in the revascularization of patients with acute ischemic stroke secondary to intracranial large vessel occlusive disease using continuous aspiration.
Potential Adverse Events
Possible complications include, but are not limited to, the following: allergic reaction and anaphylaxis from contrast media; acute occlusion; air embolism; arteriovenous fistula; death; device malfunction; distal embolization; emboli; false aneurysm formation; hematoma or hemorrhage at access site; inability to completely remove thrombus; infection; intracranial hemorrhage; ischemia; kidney damage from contrast media; neurological deficits including stroke; vessel spasm, thrombosis, dissection, or perforation.
PENUMBRA SYSTEM – Intended Use
The PENUMBRA SYSTEM is intended for use in the revascularization of patients with acute ischemic stroke secondary to intracranial large vessel occlusive disease using continuous aspiration.
Potential Adverse Events
Possible complications include, but are not limited to, the following: allergic reaction and anaphylaxis from contrast media; acute occlusion; air embolism; arteriovenous fistula; death; device malfunction; distal embolization; emboli; false aneurysm formation; hematoma or hemorrhage at access site; inability to completely remove thrombus; infection; intracranial hemorrhage; ischemia; kidney damage from contrast media; neurological deficits including stroke; vessel spasm, thrombosis, dissection, or perforation; radiation exposure that may lead to cataracts, skin reddening, burns, alopecia, or neoplasia from x-ray exposure.
PENUMBRA SYSTEM – Intended Use
The PENUMBRA SYSTEM is intended for use in the revascularization of patients with acute ischemic stroke secondary to intracranial large vessel occlusive disease using continuous aspiration.
Potential Adverse Events
Possible complications include, but are not limited to, the following: allergic reaction and anaphylaxis from contrast media; acute occlusion; air embolism; arteriovenous fistula; death; device malfunction; distal embolization; emboli; false aneurysm formation; hematoma or hemorrhage at access site; inability to completely remove thrombus; infection; intracranial hemorrhage; ischemia; kidney damage from contrast media; neurological deficits including stroke; vessel spasm, thrombosis, dissection, or perforation.
PENUMBRA ENGINE – Intended Use
The PENUMBRA ENGINE is intended as a vacuum source for Penumbra Aspiration Systems.
Penumbra Pump MAX – Intended Use
The Penumbra Pump MAX is intended as a vacuum source for the Penumbra Aspiration Systems.
