Pioneer in Aspiration
From the original Penumbra System in 2008 to today’s RED Reperfusion Catheters, Penumbra has driven the management of Large Vessel Occlusions forward in constant technological innovation. Through continuing to support clinical studies, Penumbra is helping to ensure new technologies are available to patients around the world.
DESIGNED TO ENHANCE DELIVERY IN TORTUOSITY
The Difference in Daily Practice


RED 62 Reperfusion Catheter
- 1.57 mm (.062″) Lumen: Delivers powerful aspiration
- 1.93 mm (.076″) Outer Diameter: Low profile for easy access
- Atraumatic Design: Engineered to effortlessly navigate tortuous anatomy
- 138 cm Length: To reach target vessel
- 11 material transitions: Optimised for performance in complex distal anatomy
RED 68 Reperfusion Catheter
- 1.73 mm (.068″) Lumen: Delivers powerful aspiration
- 2.13 mm (.084″) Outer Diameter: Atraumatic tip design for improved navigation
- REDglide Coating: Enhanced distal hydrophilic coating for smooth trackability
- 132 cm Length: To reach target vessel
- 16 material transitions: Optimised for performance in challenging anatomy
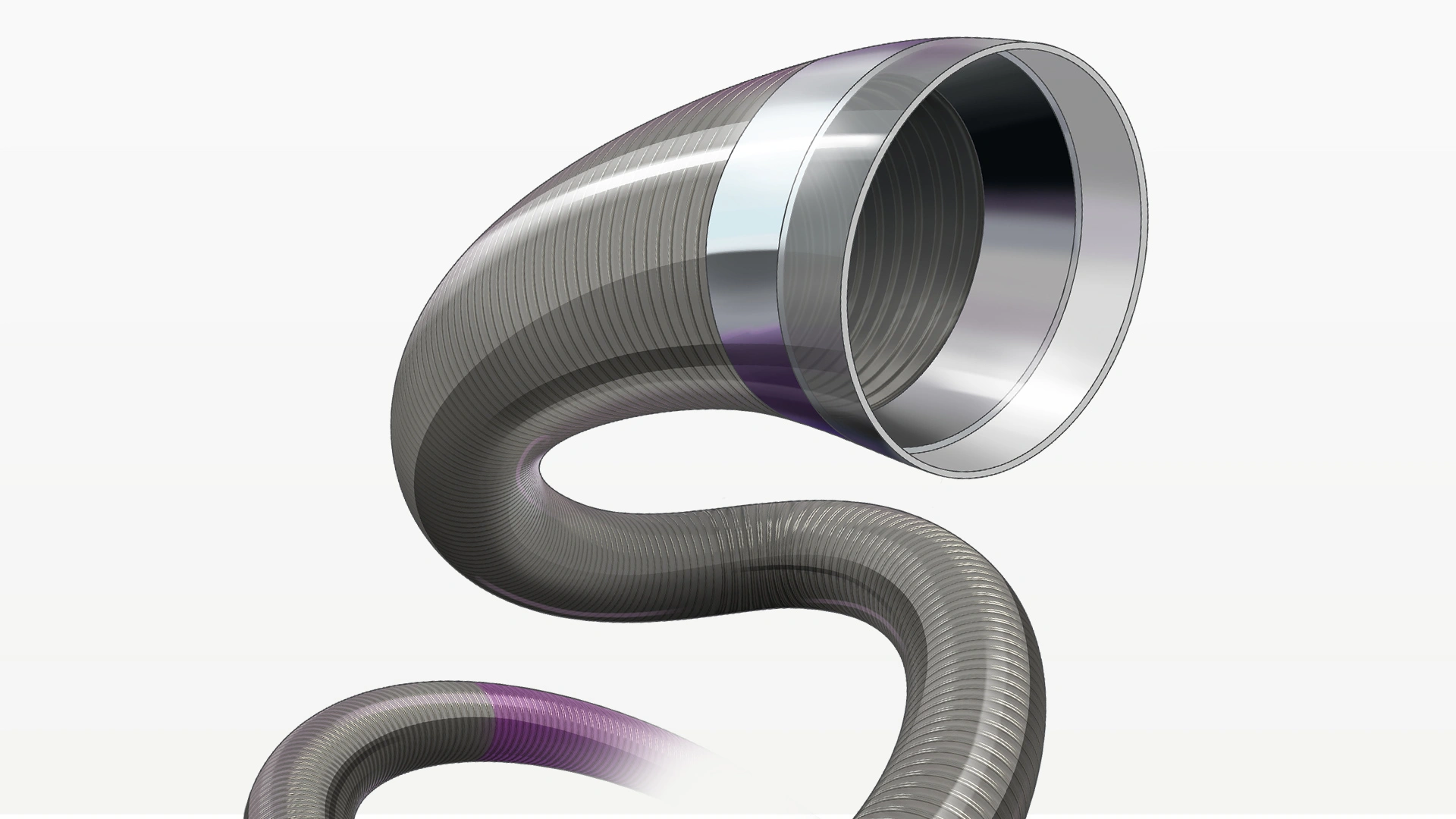

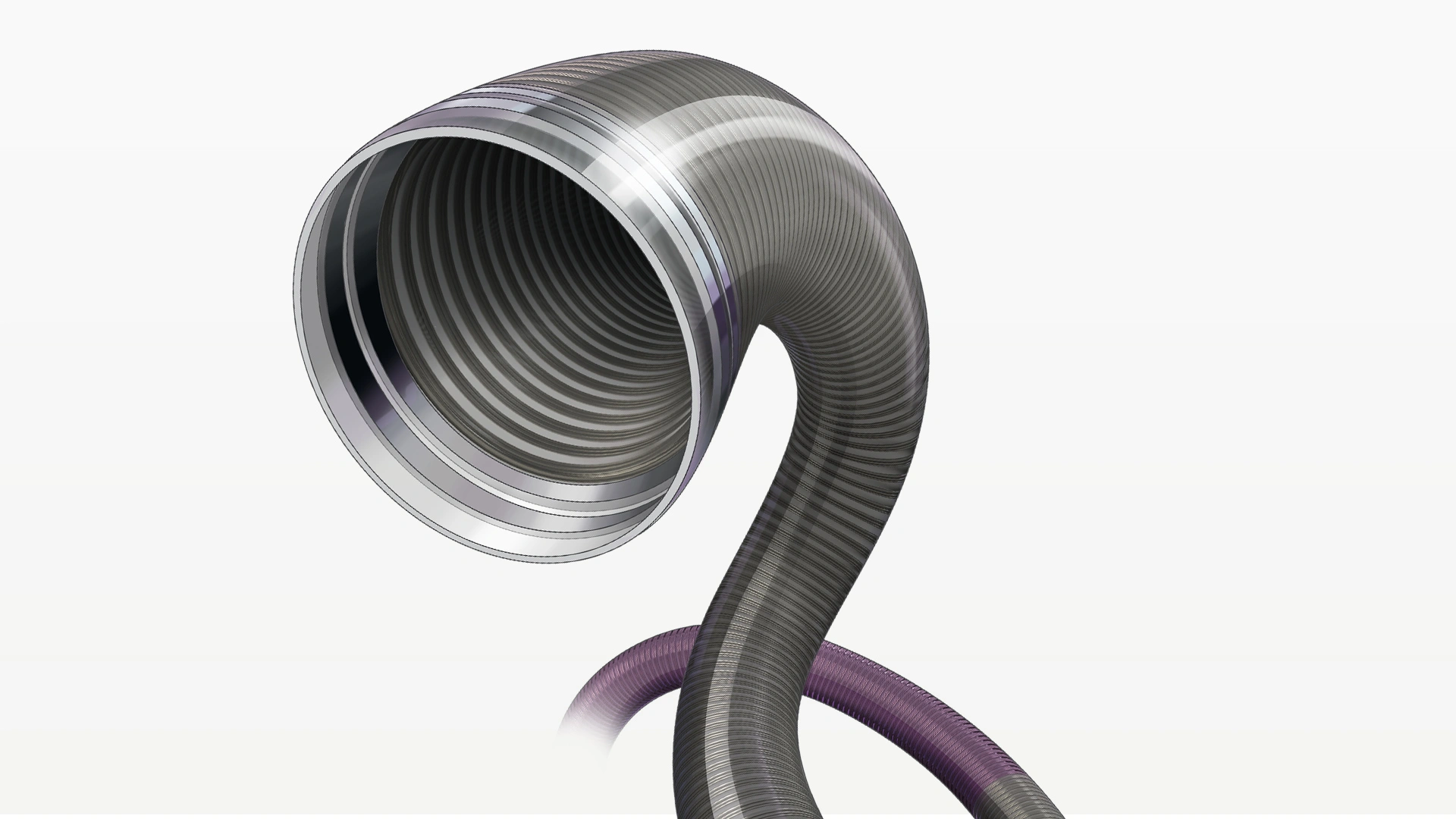

RED 72 Reperfusion Catheter
- 1.83 mm (.072″) Lumen: Delivers highest TRFa
- 2.16 mm (.085″) Outer Diameter: Articulating marker band designed to improve tip softness
- REDglide Coating: Enhanced distal hydrophilic coating for smooth trackability
- 132 cm Length: To reach target vessel
- 20 material transitions: Optimised for performance in challenging anatomy
a. As compared to AXS Vecta™ 71 and Stryker® (Medela), React™ 71 and Riptide™, AXS Vecta 74 and Stryker (Medela). Thrombus Removal Force (TRF) = (Catheter Tip Area) × (Vacuum). Sources: company website, marketing literature, IFU, and data on file.
Copyright© 2022 - 2023 Penumbra, Inc. All rights reserved. The Penumbra P logo, Penumbra RED, and Penumbra ENGINE are registered trademarks or trademarks of Penumbra, Inc. in the USA and other countries. All other trademarks are the property of their respective owners. 25607, Rev. C 05/23 EU



